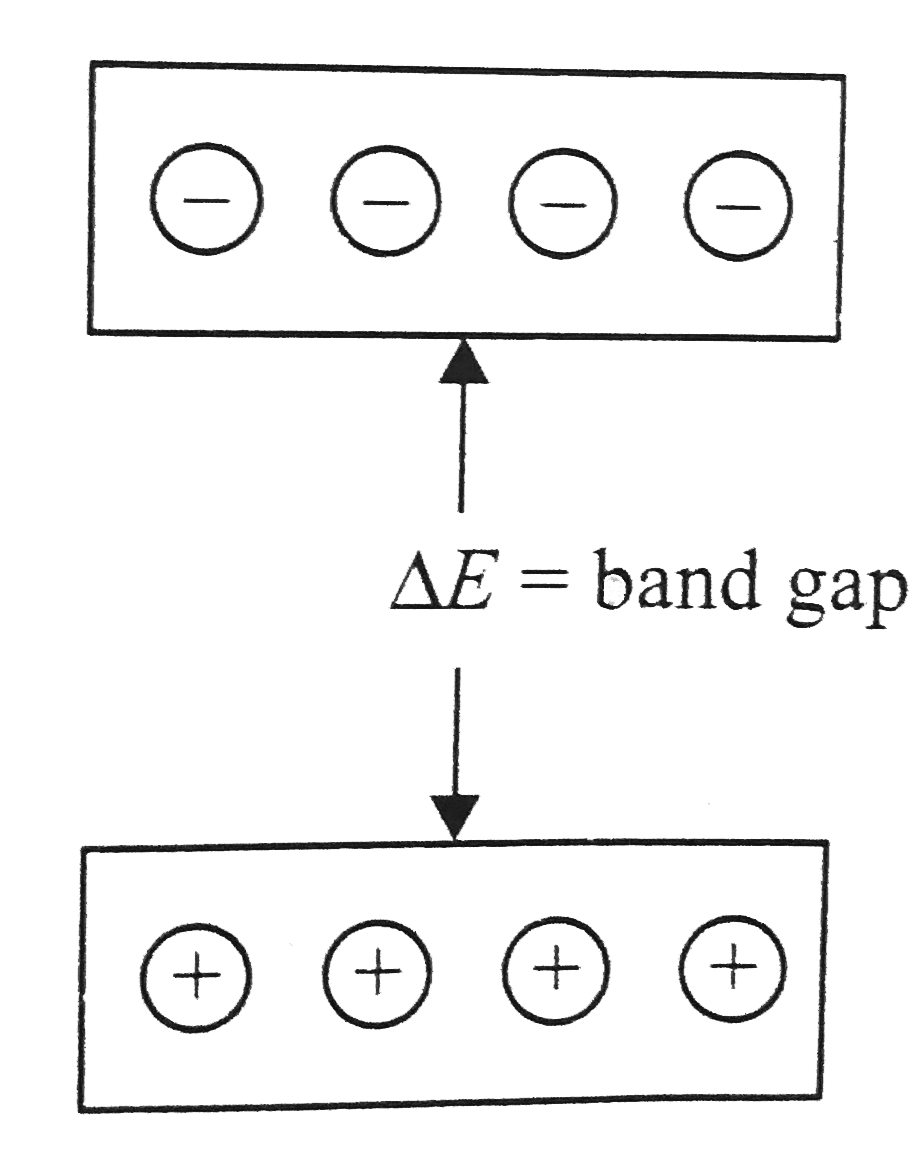
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
A2Z|Exercise Magnetism And Bragg'S Equation|13 VideosSOLID STATE
A2Z|Exercise Section B - Assertion Reasoning|21 VideosSOLID STATE
A2Z|Exercise Structure, Coordination Number, Radious Ratio Rule, Density Of Unit Cell|72 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Section D - Chapter End Test|30 VideosSOLUTIONS
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-SOLID STATE-Defects In Solids And Semiconductors
- The corrent statement regarding defects is solids in solids is
Text Solution
|
- In AgBr crystal , the ion size lies in the order Ag^(+) lt lt Br^(-) T...
Text Solution
|
- Frenkel defect is caused due to
Text Solution
|
- How many energy levels are prent in 3s conduction hand of a single cry...
Text Solution
|
- Which defect cause decrease in the density of crystal?
Text Solution
|
- To get n-type doped semiconductor, impurity to be added to silicon sho...
Text Solution
|
- The flame colours of metal ions are due to
Text Solution
|
- Schottky defect in crystal is when
Text Solution
|
- When electron are trapped into the crystal in anion cancy ,the defect ...
Text Solution
|
- In which of the following oxides conducting or insulting property of ...
Text Solution
|
- The correct statement in the following is
Text Solution
|
- The pyknometric density of NaCl crystal is 2.165 xx 10^(3 kg m^(-3) wh...
Text Solution
|
- For silicon , given Wavelength of light that excite an electron fro...
Text Solution
|
- What type of crystal defect is indicated in the diagram given below ...
Text Solution
|
- In the laboratory , sodium choride is made by burning the sodium in th...
Text Solution
|
- If NaCI is droped with 10^(-3) mole of SrCI(2) then number of cationic...
Text Solution
|
- The following is not a function an impurity present in a crystal
Text Solution
|
- The corrent statement regarding F-centre is
Text Solution
|
- If a non-metal is added to the interstitial sites of a metal, then the...
Text Solution
|
- NaCI shown Schottky defect and AgCI frenkel defect .Their elelctrical ...
Text Solution
|