A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
A2Z|Exercise Section B - Assertion Reasoning|22 VideosELECTROCHEMISTRY
A2Z|Exercise AIPMT/NEET Questions|35 VideosELECTROCHEMISTRY
A2Z|Exercise Fuel Cell And Batteries|10 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Section D - Chapter End Test|30 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF METALS
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ELECTROCHEMISTRY-Construction And Working Of A Cell, Electrochemical Series And Its Applications
- The standard reduction potential for Fe^(2+) //Fe and Sn^(2+) //Sn ele...
Text Solution
|
- The reaction is spontaneous if the cell potenital is .
Text Solution
|
- The oxdation potentiasl of following half-cell reactions are given Zn ...
Text Solution
|
- Standard electrode potential of cell H2 ||H^+ || Ag^+ | Ag is ,.
Text Solution
|
- Consider the following E^o values : E(Fe^(3+)//Fe^(2+)) = + 0.77 V ...
Text Solution
|
- For the feasibility of a redox reaction in a cell, the emf should be.
Text Solution
|
- The standared reduction potntial E^o for the half-reactions are as. ...
Text Solution
|
- The gasX at 1 atm is bubbled through a solution containing a mixture o...
Text Solution
|
- The EMF of the cell Ni |Ni|Ni^(2+) ||Cu^(2+) |Cu(s) is 0.59 volt. The...
Text Solution
|
- The standard EMF of a Daniell cell is 1.10 volt. The maximum electric...
Text Solution
|
- The standard potentials at 298 K for the following halfreactions are a...
Text Solution
|
- The standard oxidation potentials of Ze and Ag in water at 25^@C are ...
Text Solution
|
- Given that E^o values of Ag^+//Ag, K^+//K, Mg^(2+)//Mg and Cr^(3+) //C...
Text Solution
|
- Which of the following is not a function of salt bridge ?
Text Solution
|
- The following facts are availabel : 2A^(c-)+B(2) rarr 2B^(-)+A(2), ...
Text Solution
|
- MnO4^(-) + 8H^(=) + 5e^(-) rarr Mn^(2+) + 4 H2 O If H^(+) concentra...
Text Solution
|
- Saturatd solution of KNO3 is used to mke salt bridge because .
Text Solution
|
- The electrode potential becomes equal to standard electrode potential ...
Text Solution
|
- Delta G^@ of the cell reaction AgCl (s) = 1/2 H2 (g) =Ag(s) H^+ + Cl...
Text Solution
|
- The emf of given cell Pt- H2 | H^+|H2 - Pt is :
Text Solution
|
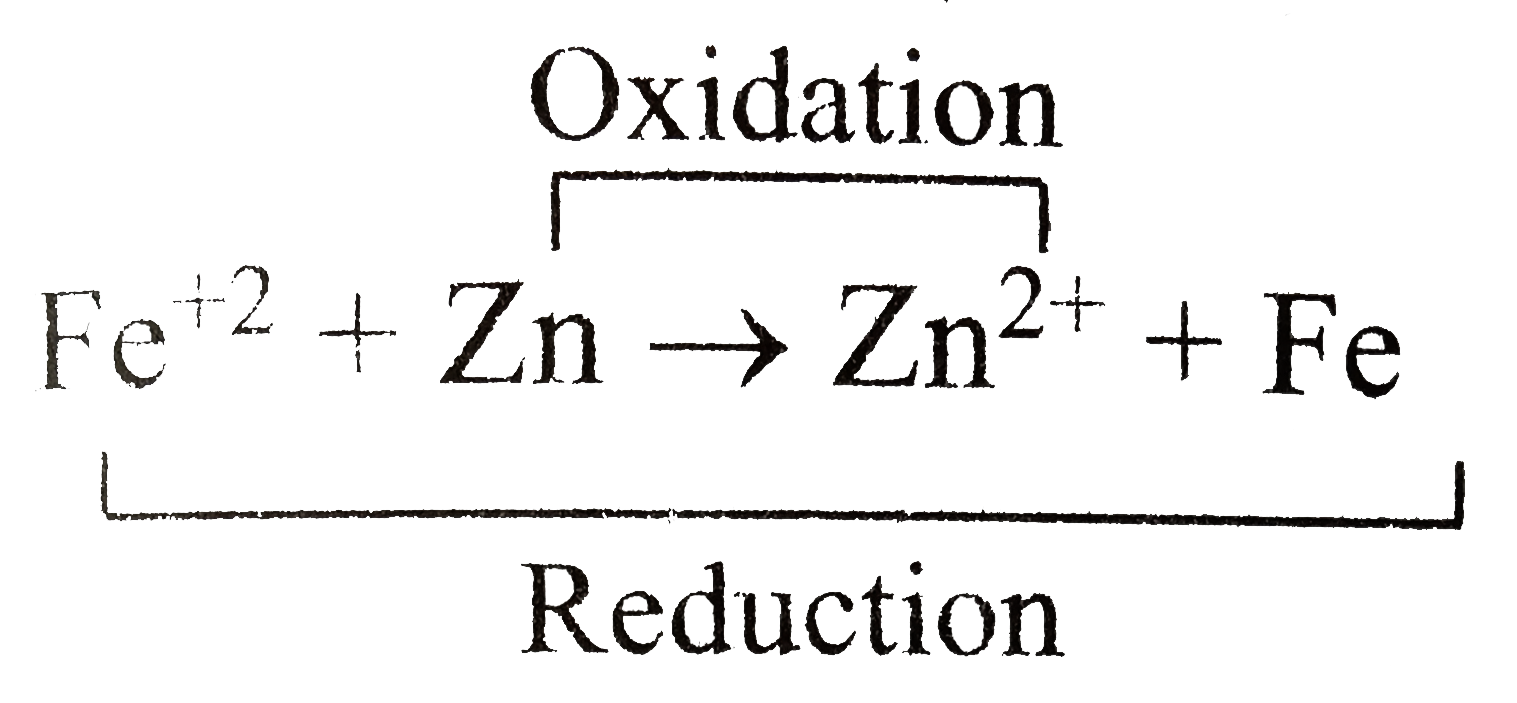 .
.