A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
A2Z|Exercise AIIMS Questions|34 VideosELECTROCHEMISTRY
A2Z|Exercise Assertion-Reasoning Questions|10 VideosELECTROCHEMISTRY
A2Z|Exercise Section B - Assertion Reasoning|22 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Section D - Chapter End Test|30 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF METALS
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ELECTROCHEMISTRY-AIPMT/NEET Questions
- The electrolytic conductance si a direct measure of .
Text Solution
|
- The emf of a Daniell cell at 298 K is E1 Zn underset((0.01M))|NnSO4 |u...
Text Solution
|
- The statdard electrode potential of the half cells is given below: Z...
Text Solution
|
- An electric current is passed through silver nitrated solution using s...
Text Solution
|
- A quantity of electrical charge that brigns about the depositiion of 4...
Text Solution
|
- A hyptherical electrochemical cell is given as A^(Ө)|A^+ (xM) || B^+ (...
Text Solution
|
- If e(Fe^(2+) //Fe)^@ =- 0. 44 1V. And E(Fe^(3+)//Fe^(2+))^(o) = 0. 771...
Text Solution
|
- The equilbrium constant for the reaction : Cu + 2 Ag^(+) (aq) rarr Cu(...
Text Solution
|
- On the basis of the folwing E^@ values, the strongest oxidizing agent ...
Text Solution
|
- Standard free energies of formation (I kJ //mol ) at 298 K are -237 ....
Text Solution
|
- The correct order of mobility of alkali metal ions in aqueous solution...
Text Solution
|
- Kohlrausch s law states that at :
Text Solution
|
- Goven : (i) CU^(2+) + 2e^- rarr Cu, E^@ = 0.337 V (ii) Cu^(2+) +e...
Text Solution
|
- Al2 O3 is reduced by electrolysis at ow potentials and high currents,...
Text Solution
|
- The correct value of emf of cell is given by (i) E("cell") = E(OP) ...
Text Solution
|
- For the reduction of silver ions with copper metal, the standard cell ...
Text Solution
|
- An increases in equivalent conductivity of strong electrolyte with dil...
Text Solution
|
- The electrode pptenticals for Cu^(2+) (aq) +e^(-) rarr Cu^+ (aq) ...
Text Solution
|
- Standard electrode potential for Sn^(4+)//Sn^(2+) couple is + 0.15 V ...
Text Solution
|
- Standared reduction electrode potenitals of three metals A, B and C ...
Text Solution
|
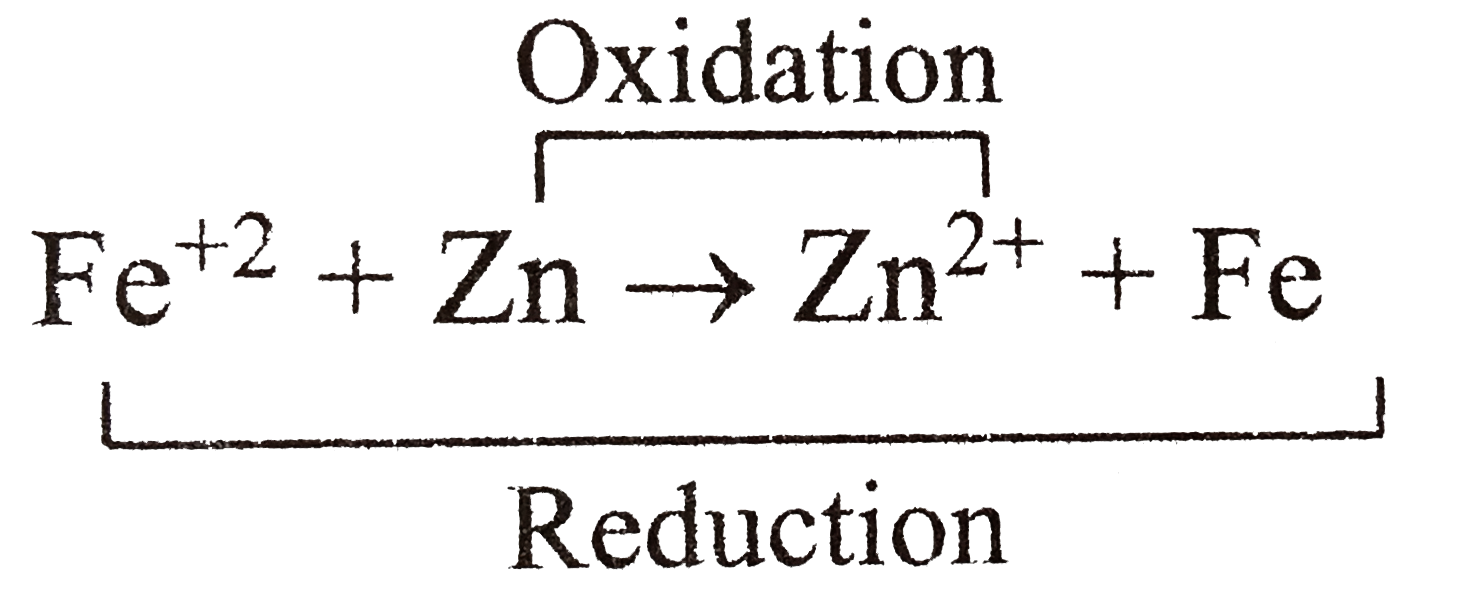 .
.