A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
A2Z|Exercise AIIMS Questions|34 VideosELECTROCHEMISTRY
A2Z|Exercise Assertion-Reasoning Questions|10 VideosELECTROCHEMISTRY
A2Z|Exercise Section B - Assertion Reasoning|22 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Section D - Chapter End Test|30 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF METALS
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ELECTROCHEMISTRY-AIPMT/NEET Questions
- Goven : (i) CU^(2+) + 2e^- rarr Cu, E^@ = 0.337 V (ii) Cu^(2+) +e...
Text Solution
|
- Al2 O3 is reduced by electrolysis at ow potentials and high currents,...
Text Solution
|
- The correct value of emf of cell is given by (i) E("cell") = E(OP) ...
Text Solution
|
- For the reduction of silver ions with copper metal, the standard cell ...
Text Solution
|
- An increases in equivalent conductivity of strong electrolyte with dil...
Text Solution
|
- The electrode pptenticals for Cu^(2+) (aq) +e^(-) rarr Cu^+ (aq) ...
Text Solution
|
- Standard electrode potential for Sn^(4+)//Sn^(2+) couple is + 0.15 V ...
Text Solution
|
- Standared reduction electrode potenitals of three metals A, B and C ...
Text Solution
|
- If E(cell)^(ɵ) for a given reaction is negative, which gives the corre...
Text Solution
|
- Standrd reduction potentials of the half reactions are given below : ...
Text Solution
|
- Limiting molar conductivity of NH4 OH I e., Lambdam (NH4 OH) is equ...
Text Solution
|
- Molar conductiveiy [Lambda m] at infinite dilution of Na CL, HCl and C...
Text Solution
|
- A button cell used in watches functions as following Zn(s) + Ag 2 O(...
Text Solution
|
- At 25^@C molar conductance of 0.1 molar qqueous solution of ammonimum...
Text Solution
|
- A hydrogen gas electrode is made by dipping platinum wire in a solutio...
Text Solution
|
- In order to completely oxidize 0.1 mol of MnO(4)^(2-) to permanganate ...
Text Solution
|
- A device that convers energy of combustion of fueles like hydrogen and...
Text Solution
|
- Aqueous solution of which of the following compounds is the best condu...
Text Solution
|
- The emf of a Daniell cell at 298 K is E1 Zn underset((0.01M))|NnSO4 |u...
Text Solution
|
- Consider the change in oxidation state of Bromine corresponding to dif...
Text Solution
|
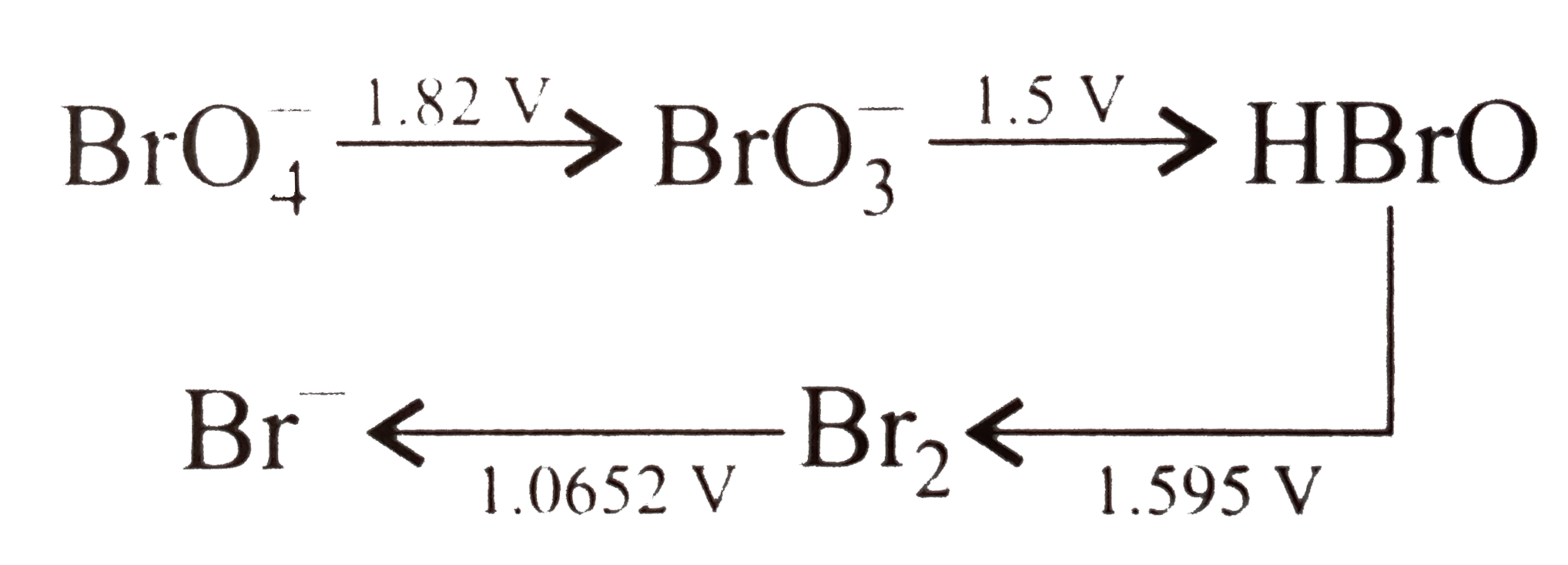 .
.