A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
A2Z|Exercise Assertion-Reasoning Questions|10 VideosELECTROCHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 VideosELECTROCHEMISTRY
A2Z|Exercise AIPMT/NEET Questions|35 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Section D - Chapter End Test|30 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF METALS
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ELECTROCHEMISTRY-AIIMS Questions
- Which of the following shows electrical conduction ?
Text Solution
|
- Which of the following statements is not applicable to electrolytic co...
Text Solution
|
- When the electric current is passed through a cell having an electroly...
Text Solution
|
- Which of the following reaction is reaction is used to make a fuel cel...
Text Solution
|
- In electrolysis of dilute H2SO4 using platinum electrodes .
Text Solution
|
- An electrochemical cell is used to convert
Text Solution
|
- Which of the following statement is true for the electrochemical Danie...
Text Solution
|
- The chemical reaction , 2 Ag Cl (s) rarr 2HCl(aq) + 2Ag(s) taking plac...
Text Solution
|
- Which one of the following metals cannot veolve H2 from acids or H2O f...
Text Solution
|
- Electode potential of Zn^(2+) //Zn is -0 . 7 V and that of Cu^(2+) /...
Text Solution
|
- Given standard electode potenitals Fe^(2+) + 2e^(-) rarr Fe, E^@ =-...
Text Solution
|
- Consider the cell reaction : Mg(s)+Cu^(2+)(aq) rarr Cu(s) +Mg^(2+)(a...
Text Solution
|
- Which of the following statements is true for fuel cells ?
Text Solution
|
- The emf of a galvanic cell, with electrode potentials of silver = + 0....
Text Solution
|
- Which of the following is highly corrosive salt ?
Text Solution
|
- The standared reduction potntial E^o for the half-reactions are as. ...
Text Solution
|
- Conductance (Siemens, S) is directly proportional to the area of the v...
Text Solution
|
- The standard emf of a cell having one electron change is found to be 0...
Text Solution
|
- On the bassis of standard electrode potential of redox couples given b...
Text Solution
|
- Celll equation : A = 2B^(+) rarr A^(2+) + 2 B A^(2+) + 2e rarr A E...
Text Solution
|
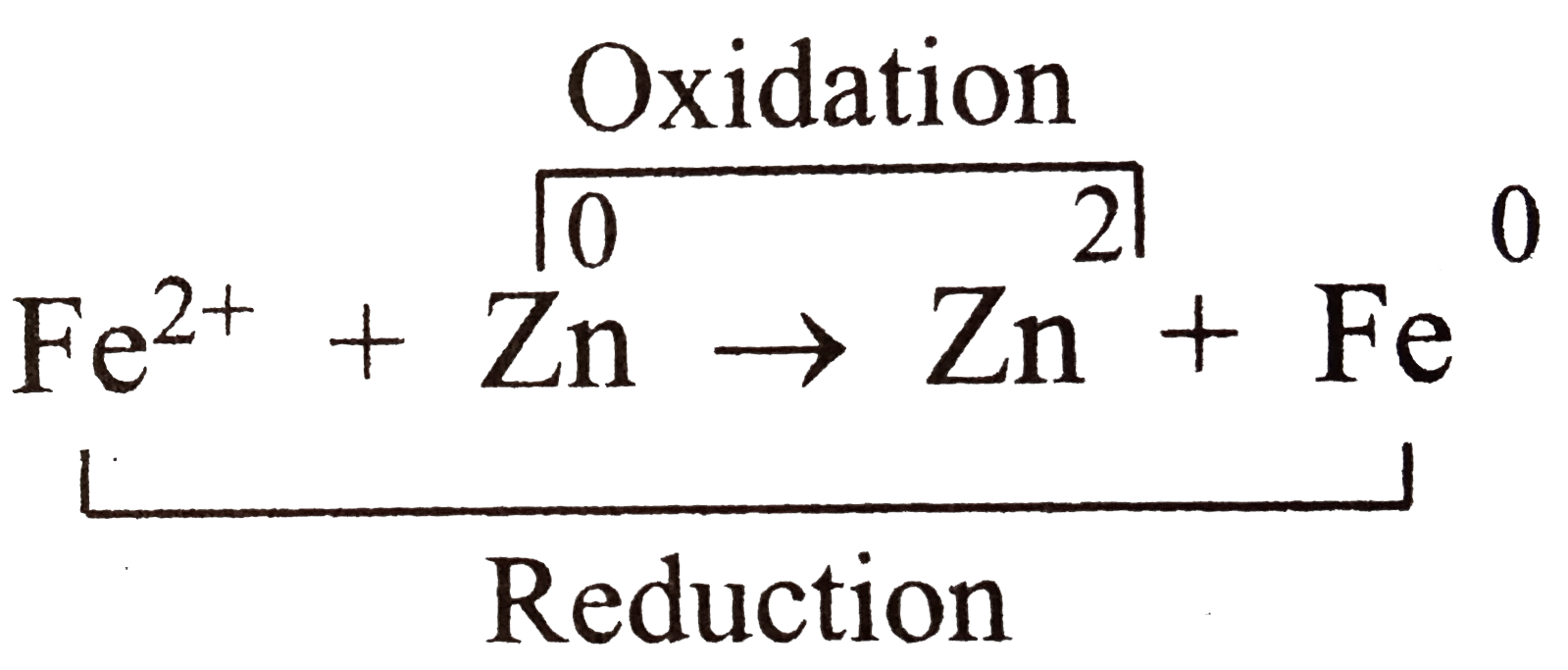 .
.