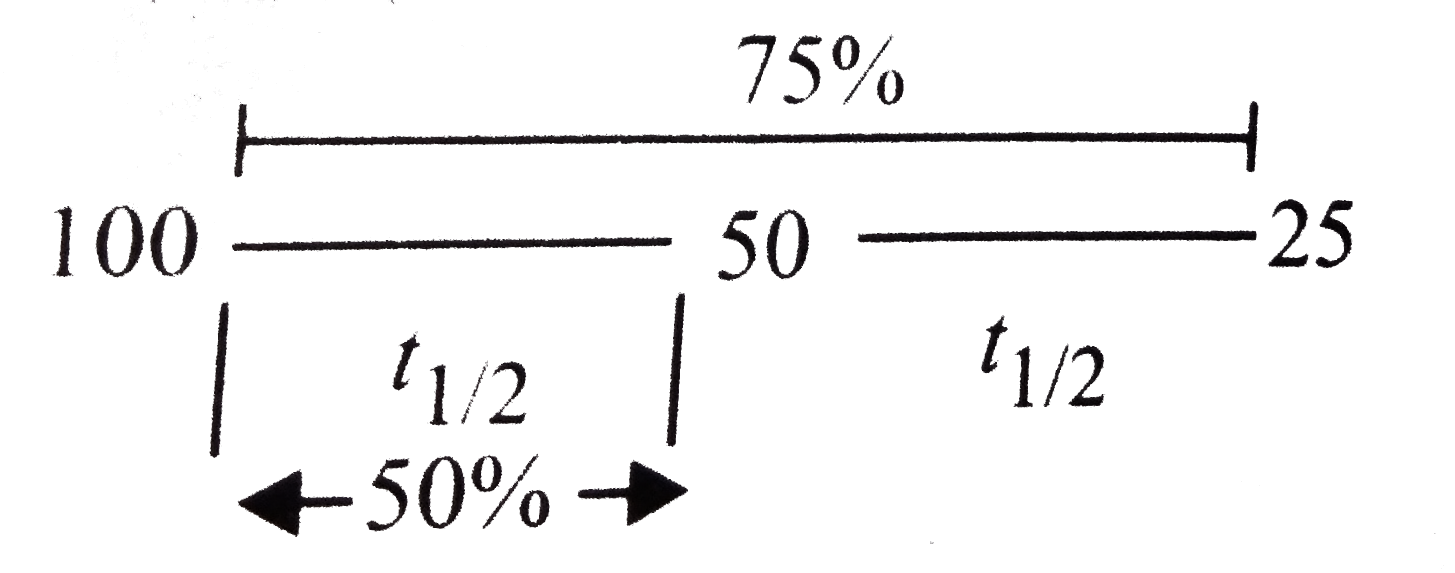A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
A2Z|Exercise AIPMT/NEET Questions|43 VideosCHEMICAL KINETICS
A2Z|Exercise AIIMS Questions|24 VideosCHEMICAL KINETICS
A2Z|Exercise Parallel Reaction, Consecutive Reaction, Special Cases Of First-Order Reactions|25 VideosBIOMOLECULES
A2Z|Exercise Section D - Chapter End Test|30 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL KINETICS-Section B - Assertion Reasoning
- Assertion: Half-life of a certain radioactive element is 100 days. Aft...
Text Solution
|
- Assertion: Time taken for the completion of 75% of a Ist order reactio...
Text Solution
|
- Assertion (A) : If the activation energy of a reaction is zero, temper...
Text Solution
|
- Assertion: For a reaction A(g) rarr B(g) -r(A) = 2.5 P(A) at 400 K ...
Text Solution
|
- Assertion: In A overset(k(1))rarr B overset(k(2))rarr C If half-life...
Text Solution
|
- Assertion: Larger is the activation energy, lesser is the effect of a ...
Text Solution
|
- Assertion: For a free radical combination, K = A. Reason: E(a) is ze...
Text Solution
|
- Assertion: Every collision of reactant molecule is not successful. R...
Text Solution
|
- Assertion: A plot of log-log(dX)/(d t) vs. log(a-X) leads to value of ...
Text Solution
|
- Assertion: the plots of log t(1//2) vs. log[A](0) gives a straight lin...
Text Solution
|
- Assertion: Emission of light as result of exposure of P to air in nigh...
Text Solution
|
- Assertion: In the reaction N(2) + 3H(2) rarr 2NH(3), the rate of react...
Text Solution
|
- Assertion (A) : The rate constant of a zero order reaction has same un...
Text Solution
|
- Assertion: In a zero order reaction, if concentration of the reactant ...
Text Solution
|
- Assertion (A) : The rate constant of a pseudo unimolecular reaction ha...
Text Solution
|
- Assertion: 50% of reaction is completed in 50 sec, therefore, 75% of t...
Text Solution
|
- Assertion: For a first order reaction, the concentration of the reacta...
Text Solution
|
- Assertion: Temperature coefficients of most of the reactions lies betw...
Text Solution
|
- Assertion: The molecularity of the reaction H(2) + Br(2) rarr 2HBr is ...
Text Solution
|
- Assertion: For the reaction RCl + NAOH(aq) rarr ROH + NACl, the rate o...
Text Solution
|