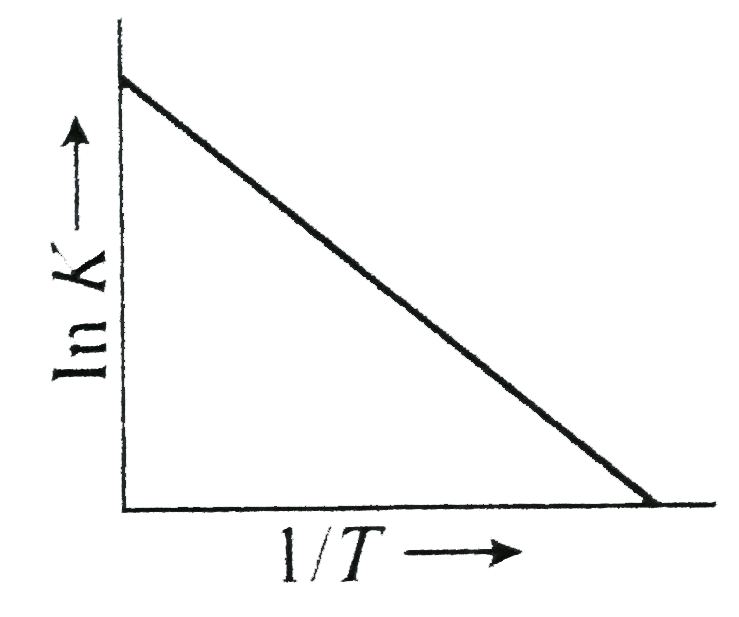
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
A2Z|Exercise Assertion-Reasoning Questions|6 VideosCHEMICAL KINETICS
A2Z|Exercise Section D - Chapter End Test|30 VideosCHEMICAL KINETICS
A2Z|Exercise AIPMT/NEET Questions|43 VideosBIOMOLECULES
A2Z|Exercise Section D - Chapter End Test|30 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL KINETICS-AIIMS Questions
- Which of the following rate laws has an overall order of 0.5 for react...
Text Solution
|
- For a chemical reaction A rarr B, it is found that the rate of reacti...
Text Solution
|
- The inversion of cane sugar is represented by C(12)H(22)O(11) + H(2)...
Text Solution
|
- A first-order reaction which is 30% complete in 30 minutes has a half-...
Text Solution
|
- For the reaction H(2) + Cl(2) overset("Sunlight")(rarr) 2HCl taking pl...
Text Solution
|
- The rate constant of a reaction is 0.69 xx 10^(-1) and the initial con...
Text Solution
|
- The rate constant of a first-order reaction is 3 xx 10^(-6) per second...
Text Solution
|
- A first-order reaction is half completed in 45 minutes. How long does ...
Text Solution
|
- A subtance 'A' decomposes by a first-order reaction starting initially...
Text Solution
|
- For reaction aA rarr xP, when [A] = 2.2 mM, the rate was found to be 2...
Text Solution
|
- If a substance with hlaf life 3 days is taken at other place in 12 day...
Text Solution
|
- An endothermic reaction with high activation energy for the forward re...
Text Solution
|
- The rate cnstant k, for the reaction N(2)O(5)(g) rarr 2NO(2)(g) + (1)...
Text Solution
|
- In the Arrhenius for a certain reaction, the value of A and E(a) (acti...
Text Solution
|
- A first order reaction is 50% complete in 30 minutes at 27^(@)C and in...
Text Solution
|
- The half-life period of a radioactive element is 140 days. After 560 d...
Text Solution
|
- The chemical reaction, 2O(3) rarr 3O(2) proceeds as follows Step1 : ...
Text Solution
|
- The temperature dependence of a reaction is represented by the Arrheni...
Text Solution
|
- Which of the following option is valid for zero reaction?
Text Solution
|
- If reaction A and B are given with same temperature and same concentra...
Text Solution
|