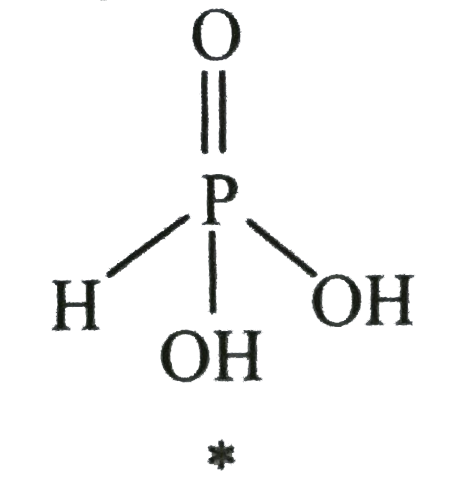
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Physical And Chemical Properties (Group 16)|20 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Compound Of Oxygen (Group 16)|28 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Compounds Of Nitrogen (Group15)|40 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Section D - Chapter End Test|30 VideosSOLID STATE
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-P BLOCK ELEMENTS ( GROUP 15,16,17,18)-Compounds Of Phosphorus (Group15)
- Write the missing product in the following reaction
Text Solution
|
- Phosphorus is manufactred by heating in an electric furnace a mixture ...
Text Solution
|
- Dissociatuion of H(3)PO(4) occurs in following stages
Text Solution
|
- Which of the following acid exist in polymeric form?
Text Solution
|
- If phospheric acid is allowed to react with sufficient quantity of NaO...
Text Solution
|
- One of the acid listed below is formed P(2)O-(3) and the rest are form...
Text Solution
|
- P(2)O(5) is heated with water to give
Text Solution
|
- Hypophosphorus acid is
Text Solution
|
- PCI(3) reacts with water to yield
Text Solution
|
- H(3)PO(3) is
Text Solution
|
- Oxidation state of +1 for phosphorus is found in
Text Solution
|
- By the action of hot conc. H(2)SO(4), phosphorus changes to
Text Solution
|
- The number of hydroxyl group in pyrophosphoric acid is
Text Solution
|
- Sodium hydroxide solution reacts with phosphorus to give phosphine. To...
Text Solution
|
- Solid PCI(5) exits as
Text Solution
|
- Which is true with regard to the properties of PH(3)?
Text Solution
|
- The number of P-O-P bridge in the structure of phosphorous pentoxide a...
Text Solution
|
- A solution of sodium metal in liquid ammonia is strongly reducing due ...
Text Solution
|
- Which of the following compound is tribasic acid?
Text Solution
|
- One mole of calciium phosphide on reaction with excess water gives
Text Solution
|