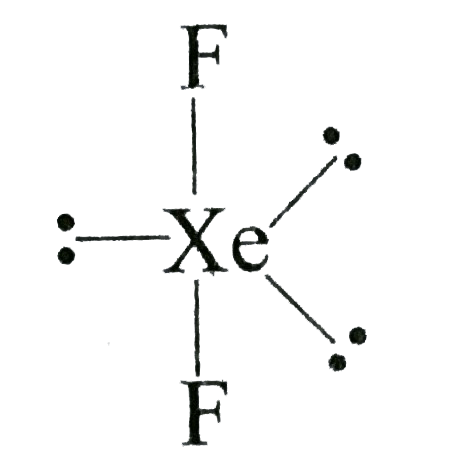
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Section B - Assertion Reasoning|35 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise AIPMT/NEET Questions|71 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Oxides, Oxoacids, Polyhalides Ions, Pseudohalides And Interhalogen Compounds|26 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Section D - Chapter End Test|30 VideosSOLID STATE
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-P BLOCK ELEMENTS ( GROUP 15,16,17,18)-General Properties And Fluorides Of Xenon (Group 18)
- Nuclear fusion produces
Text Solution
|
- The fluoride which does not exist is
Text Solution
|
- XeF(2) molecule is
Text Solution
|
- Electron affinity for a noble gas is approximately equal to
Text Solution
|
- Which of the noble gases is the least polarized?
Text Solution
|
- Which one of the following noble gases is not found in atmoshphere?
Text Solution
|
- Which cahracteric of zero group element is common?
Text Solution
|
- XeF(6) on complete hydrolysis gives
Text Solution
|
- Boiling point is more for
Text Solution
|
- Which of the following noble gases does not form clathrate compounds?
Text Solution
|
- Xenon best reacts with
Text Solution
|
- The compound that attacks pyrex glass is
Text Solution
|
- XeF(6) on reaciton with KF yields
Text Solution
|
- XeF(4) reacts with SF(4) to give
Text Solution
|
- The oxidation state of Pt in Xe^(+)[Ptf(6)]^(-) is
Text Solution
|
- The idea which prometed Bartlett to prepare first ever compound of nob...
Text Solution
|
- A radioactive element X-decays to give two inert gases. X is
Text Solution
|
- If two litres of aitr is passed repectedly over heated copper and heat...
Text Solution
|
- The van der waals forces are the greatest in
Text Solution
|
- In XeF(2).2SbF(5)
Text Solution
|