A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Section B - Assertion Reasoning|35 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise AIPMT/NEET Questions|71 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Oxides, Oxoacids, Polyhalides Ions, Pseudohalides And Interhalogen Compounds|26 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Section D - Chapter End Test|30 VideosSOLID STATE
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-P BLOCK ELEMENTS ( GROUP 15,16,17,18)-General Properties And Fluorides Of Xenon (Group 18)
- If two litres of aitr is passed repectedly over heated copper and heat...
Text Solution
|
- The van der waals forces are the greatest in
Text Solution
|
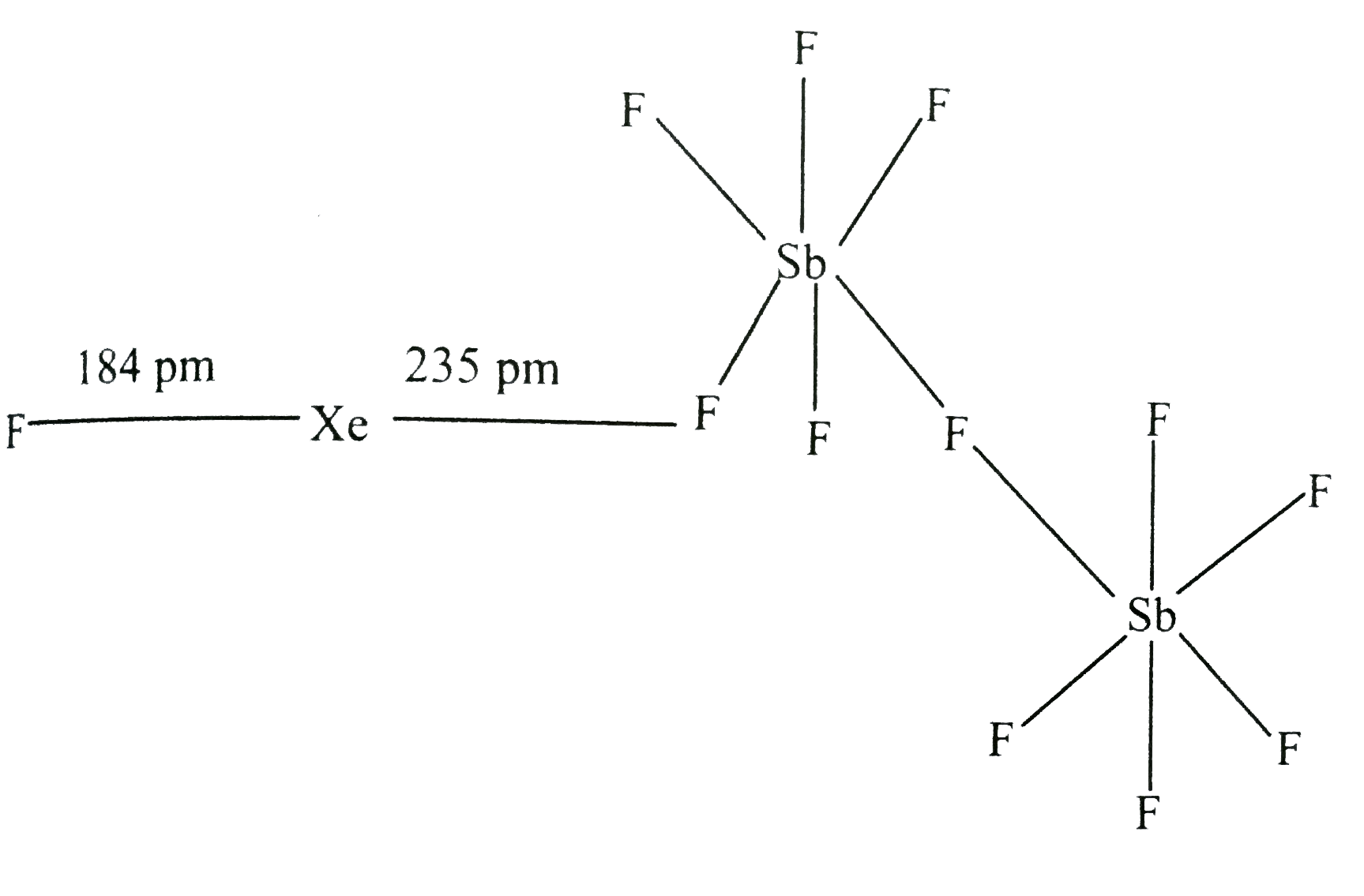
- In XeF(2).2SbF(5)
Text Solution
|
- The poisson's ratio for inert gases is:
Text Solution
|
- The none-existent species is
Text Solution
|
- Noble gases can be separated by:
Text Solution
|
- The noble gas which behaves abnormally in liquid state is
Text Solution
|
- The ease of liquefation of noble gases decrease in the order:
Text Solution
|
- The formation of O(2)^(+)[PtF(6)]^(-) is the basis for the formation o...
Text Solution
|
- Out of (i)XeO(3)(ii)XeO(2)F(2) and (iii)XeO(4), the molecules having s...
Text Solution
|
- [HXeO(4)]^(-)+Ohrarr[X]+[Y]+O(2)+H(2)O The products [X] and [Y] in u...
Text Solution
|
- The oxidation number of xenon in XeOF(2) is
Text Solution
|
- When a solution of XeO(3) is treated with metal fluoride, the product ...
Text Solution
|
- Among the following molecules, (i)XeO(3)(ii)XeOF(4)(iii)XeF(6) those h...
Text Solution
|
- Helium is used in gas balloon instead of hydrogen because
Text Solution
|
- [HXeO(4)]^(-)+OH^(-)rarr[X]+[Y]+O(2)+H(2)O The products [X] and [Y] ...
Text Solution
|
- In XeO(3),Xe is
Text Solution
|
- In Kroll and Icl process of the production of titaninum, the inert gas...
Text Solution
|
- Match the shape to the formula. Which pairing is incorrect?
Text Solution
|
- Helium gives a characteristic spectrum with:
Text Solution
|