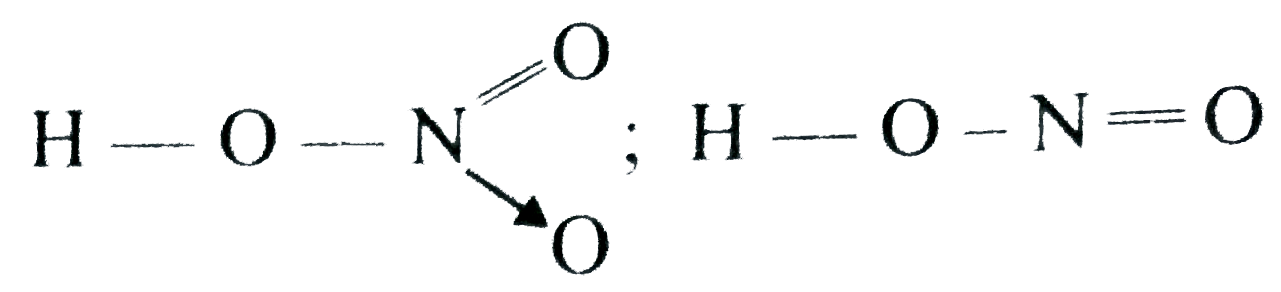A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise AIPMT/NEET Questions|71 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise AIIMS Questions|25 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise General Properties And Fluorides Of Xenon (Group 18)|44 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Section D - Chapter End Test|30 VideosSOLID STATE
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-P BLOCK ELEMENTS ( GROUP 15,16,17,18)-Section B - Assertion Reasoning
- Asseration:Although:Although PF(5),PCl(5) and PBr(5) are known, the pe...
Text Solution
|
- HNO3 is a stronger acid than HNO2 In HNO3, there are two nitrogen to...
Text Solution
|
- Asseration: NH(3) can be dried by CaCl(2). Reason: CaCl(2) is a good...
Text Solution
|
- Asseration: lowest oxidation number of N-family is -3 and highest oxid...
Text Solution
|
- Asseration: Nitrogen forms H(2)N-NH(2),N(2) and N(3)^(-) ions whereas ...
Text Solution
|
- Asseration: The abnormality in b.pt. of NH(3) in hydrides of N-family ...
Text Solution
|
- Asseration: The halogens develop+ve charge on N-atom and thus more +ve...
Text Solution
|
- Asseration: N in NO(2) and nitrolic acid has +4 and +3 oxidation numb...
Text Solution
|
- Asseration: Number of P-O-P bonds in cyclotrimetaphosphoric acid is 3....
Text Solution
|
- Asseration: NH(3) and PH(3) differ from each other in their reaction w...
Text Solution
|
- Asseration: The elctronic structure of O(3) is: Reason: structur...
Text Solution
|
- Assertion: Superoxide ion is paramagnetic whereas peroxide ion is diam...
Text Solution
|
- Assertion: Both are dibasic and permonosulphuric acid does not exit in...
Text Solution
|
- Asseration: The thermal stability of hydrides of oxygen family decreas...
Text Solution
|
- Asseration: Number of S-S bonds in H(2)S(n)O(6) is (n-1). Reason: H(...
Text Solution
|
- Asseration: 3SnCl(2)+6HCl+O(3)rarr3SnCl(4)+3H(2)O is possible reaction...
Text Solution
|
- Asserarion:Bleaching action action of SO(2) is temporary and by reduct...
Text Solution
|
- Asseration: Cyclic trimer of SO(3) possesses have six membered hetero-...
Text Solution
|
- Assertion: F atom has less negative electron gain enthaply than Ci ato...
Text Solution
|
- Asseration: F-F bond in F(2) molecule is strong. Reason: F-atom is s...
Text Solution
|