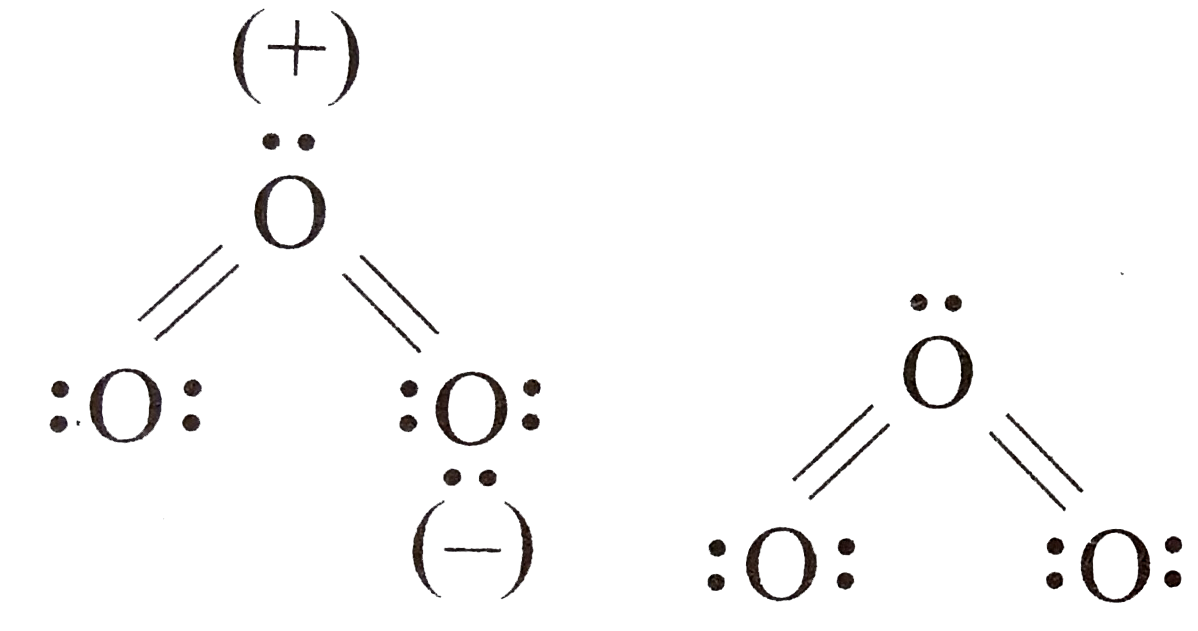
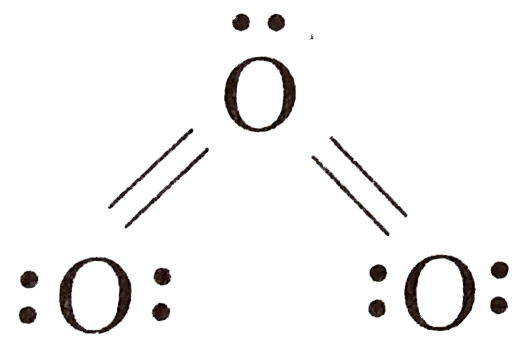
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise AIPMT/NEET Questions|71 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise AIIMS Questions|25 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise General Properties And Fluorides Of Xenon (Group 18)|44 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Section D - Chapter End Test|30 VideosSOLID STATE
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-P BLOCK ELEMENTS ( GROUP 15,16,17,18)-Section B - Assertion Reasoning
- Asseration: Number of P-O-P bonds in cyclotrimetaphosphoric acid is 3....
Text Solution
|
- Asseration: NH(3) and PH(3) differ from each other in their reaction w...
Text Solution
|
- Asseration: The elctronic structure of O(3) is: Reason: structur...
Text Solution
|
- Assertion: Superoxide ion is paramagnetic whereas peroxide ion is diam...
Text Solution
|
- Assertion: Both are dibasic and permonosulphuric acid does not exit in...
Text Solution
|
- Asseration: The thermal stability of hydrides of oxygen family decreas...
Text Solution
|
- Asseration: Number of S-S bonds in H(2)S(n)O(6) is (n-1). Reason: H(...
Text Solution
|
- Asseration: 3SnCl(2)+6HCl+O(3)rarr3SnCl(4)+3H(2)O is possible reaction...
Text Solution
|
- Asserarion:Bleaching action action of SO(2) is temporary and by reduct...
Text Solution
|
- Asseration: Cyclic trimer of SO(3) possesses have six membered hetero-...
Text Solution
|
- Assertion: F atom has less negative electron gain enthaply than Ci ato...
Text Solution
|
- Asseration: F-F bond in F(2) molecule is strong. Reason: F-atom is s...
Text Solution
|
- Asseration: HF forms two series of salts but HCl not. Reason: F-atom...
Text Solution
|
- Asseration: Iodine does not displace Cl(2) or Br(2) from their chlorid...
Text Solution
|
- Asseration: A fresh stain of iodine is washed with hypo solution. Reas...
Text Solution
|
- Asseration: Liquid I(2) conducts current very slightly. Reason: Iodi...
Text Solution
|
- Asseration: Liquid HF is used as non-aqueous solvent and many acid-bas...
Text Solution
|
- Asseration: Mineral acids on dissolving in liquid HF acts like a base....
Text Solution
|
- Asseration: The reaciton between HClO(4) and liquid HF is: HClO(4)+HFr...
Text Solution
|
- Asseration: ClO(2) possess old number of electrons. Reason: ClO(2) d...
Text Solution
|