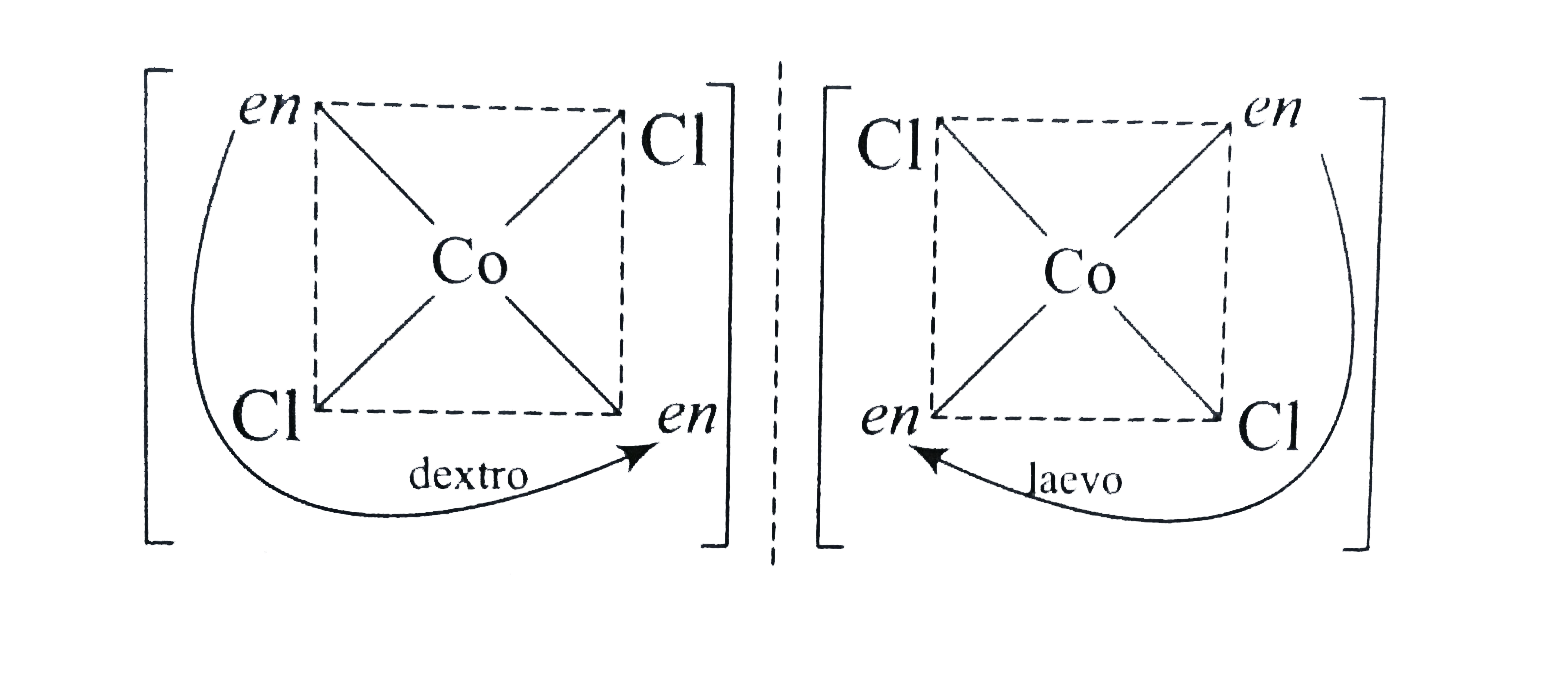
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
A2Z|Exercise AIIMS Questions|17 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Assertion-Reasoning Questions|5 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Section B - Assertion Reasoning|15 VideosCHEMICAL KINETICS
A2Z|Exercise Section D - Chapter End Test|30 VideosELECTROCHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-COORDINATION COMPOUNDS-AIPMT/NEET Questions
- Which of the following does not have a metal carbon bond?
Text Solution
|
- Which of the following is considered to be an anticancer species ?
Text Solution
|
- Which one of the following is expected to exhibit optical isomerism (e...
Text Solution
|
- Which one of the following is an inner orbital complex as well as diam...
Text Solution
|
- [Cr(H2 O)6]Cl3 (at no. of Cr = 24) has a magnetic moment of 3.83 B.M. ...
Text Solution
|
- [Co(NH2)2]Cl exhibits
Text Solution
|
- The d- electron configurations of Mn^(2+),Fe^(2+),Co^(3+) and Ni^(2+) ...
Text Solution
|
- Which of the following will give a pair of enantiomorphs? .
Text Solution
|
- Which of the following complexes exhibits the highest paramagnetic beh...
Text Solution
|
- Among TiF(6)^(2-), CoF(6)^(3-), Cu(2)C1(2) and NiC1(4)^(2-) (At. No. T...
Text Solution
|
- Which of the following complexes is not expected to exhibit isomerism?
Text Solution
|
- [Co(NH3)4Cl2]^(+) exhibits
Text Solution
|
- Which of the following complex ion(s) is/are not expected to absorb vi...
Text Solution
|
- Which of the following does not have optical isomer?
Text Solution
|
- Crystal field stabilization energy for high spin d^4 octahedral comple...
Text Solution
|
- Which of the following complex ion is not expected to absorb visible l...
Text Solution
|
- The complex, [Pt(py)(NH3)BrCl] will have how many geometrical isomers?
Text Solution
|
- [Co(HN(3))(6)][Cr(CN)(6)] and [Cr(NH(3))(6)][Co(CN)(6)] are .
Text Solution
|
- The d-electron configurations of Cr^(2+), Mn^(2+), Fe^(2+) and Co^(2+)...
Text Solution
|
- Of the following complex ions, which is diamagnetic in natures?
Text Solution
|