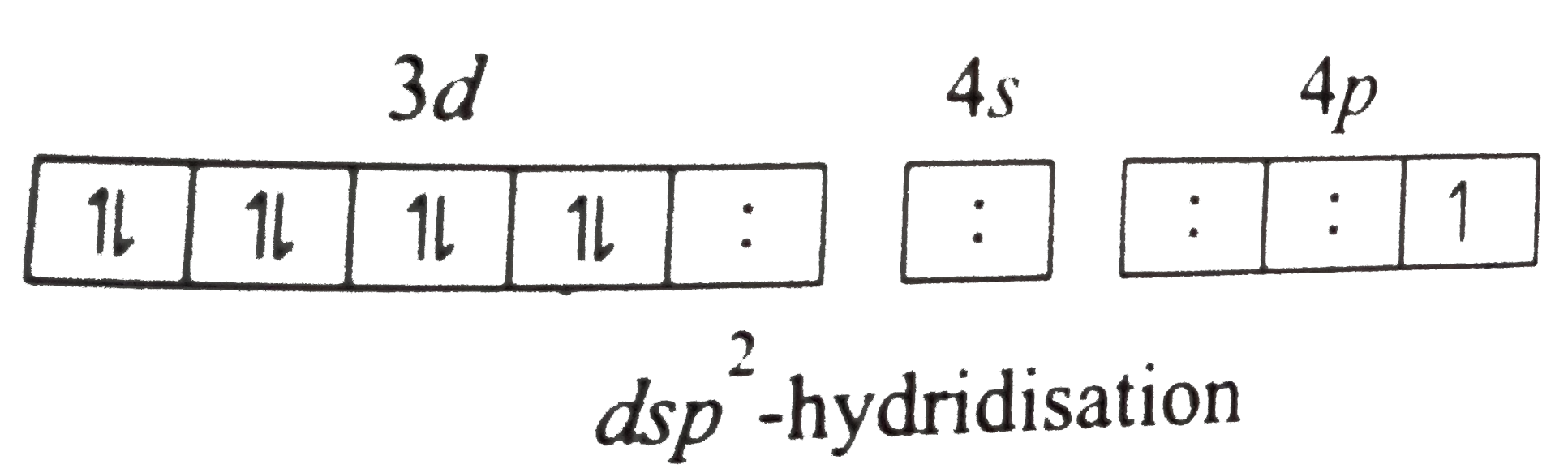A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
A2Z|Exercise AIIMS Questions|17 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Assertion-Reasoning Questions|5 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Section B - Assertion Reasoning|15 VideosCHEMICAL KINETICS
A2Z|Exercise Section D - Chapter End Test|30 VideosELECTROCHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-COORDINATION COMPOUNDS-AIPMT/NEET Questions
- Which one of the following is an outer orbital complex and exhibits pa...
Text Solution
|
- Low spin complex of d^6-cation in an octahedral field will have the fo...
Text Solution
|
- A magnetic moment of 1.73 B.M. will be shown by one among the followin...
Text Solution
|
- An excess of AgNO3 is added to 100mL of a 0.01M solution of dichlorote...
Text Solution
|
- Among the following complexes, the one which shows zero crystal field ...
Text Solution
|
- The complex used as an anticancer agent is
Text Solution
|
- Cobalt (III) chloride forms several octahedral complexes with amonia. ...
Text Solution
|
- Which of these statements about [Co(CN)6]^(3-) is true?
Text Solution
|
- The IUPAC name of the coordination compound K3[Fe(CN)6] is:
Text Solution
|
- Both [Ni(CO)4] and [Ni (CN)4]^(2-) are diamagnetic The hybridisation...
Text Solution
|
- The sum of coordination number and oxidation number of the metal M in ...
Text Solution
|
- What is the co-ordination number of the metal in [Co(en)2Cl2]^+?
Text Solution
|
- Which of the following has longest C-O bond length? (Free C-O bond len...
Text Solution
|
- An example of a sigma bonded organometallic compound is:
Text Solution
|
- The correct order of the stoichiometries of AgCl formed when AgNO3 in ...
Text Solution
|
- Correct increasing order for the wavelengths of absorption in the visi...
Text Solution
|
- Pick out the correct statement with respect to [Mn(CN)(6)]^(3-):
Text Solution
|
- The type of isomersim shown by the complex [CoCl(2) (en)(2)] is
Text Solution
|
- The geometry and magnetic behaviour of the complex [Ni(CO)4] are
Text Solution
|
- Iron carbonyl, Fe(CO)5 is
Text Solution
|