A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING HALOGENS
A2Z|Exercise Chemical Properties Of Haloarenes|31 VideosORGANIC COMPOUNDS CONTAINING HALOGENS
A2Z|Exercise Section B - Assertion Reasoning|16 VideosORGANIC COMPOUNDS CONTAINING HALOGENS
A2Z|Exercise Elimination Reaction And Polyhalogen Compounds|67 VideosMOCK TEST
A2Z|Exercise Mock Test 2|45 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ORGANIC COMPOUNDS CONTAINING HALOGENS-Methods Of Preparationof Haloarenes
- In the above process product A is .
Text Solution
|
- X,Y and Z have the value of mu as 1.78,1.9 and 1.3D respectively Whi...
Text Solution
|
- Chlorobenzene is prepared commercially by
Text Solution
|
- Consider the following reaction Thyroxine a thyroid hormone that hel...
Text Solution
|
- C(6)H(6)+CI(2)overset(UVLight)rarr Product In above reaction product i...
Text Solution
|
- Dazonium salts +Cu(2)CI(2)+HCIrarr the reaction is known as .
Text Solution
|
- m-Bromotoluene is prepared by
Text Solution
|
- Toluene reacts with excess of CI(2) in presence of sunlight to give a...
Text Solution
|
- Which of the following will best convert nitobenzene into 3-fluorobrom...
Text Solution
|
- m-Bromotoluene is prepared by
Text Solution
|
- The raction of toluene with CI(2) in presence of FeCI(3) gives X and r...
Text Solution
|
- Which one among the following compounds has the highest dipole moment ...
Text Solution
|
- Fluorobenzene (C(6)H(5)F) can be synthesized in the laboratory ,
Text Solution
|
- The reaction of toluene with CI(2) in presence of FeCI(3) gives predom...
Text Solution
|
- Chlorobenzene can be prepared by reacting aniline with
Text Solution
|
- The reaction of biphenyl with HOCI in the presence of a strong acid gi...
Text Solution
|
- The reaction of toluene with CI(2) in presence of FeCI(3) gives predom...
Text Solution
|
- On treatment with chlorine in presence of sunlight toluene giv. Es the...
Text Solution
|
- When the all-cis isomer of C(6)H(6)CI(6)(1,2,3,4,,5,6-Hexachlorocycloh...
Text Solution
|
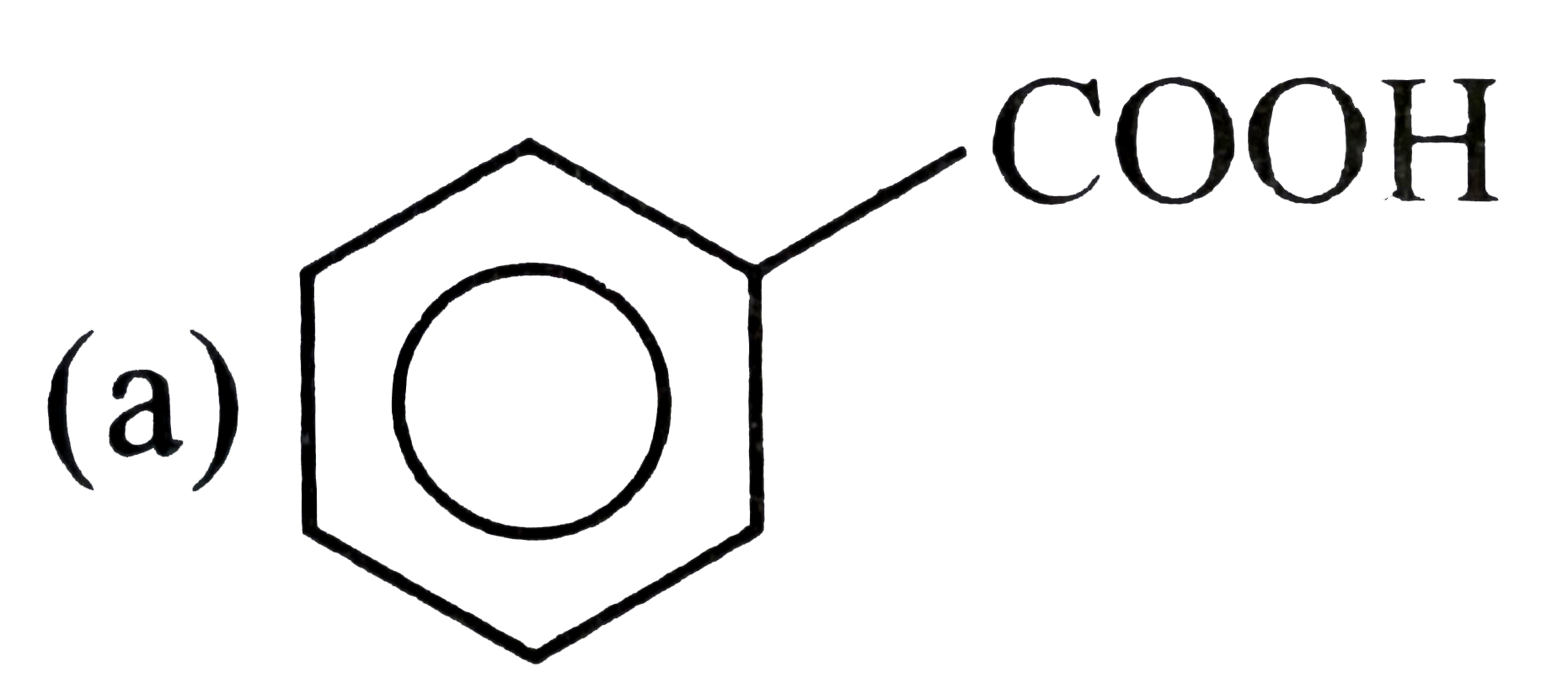 .
.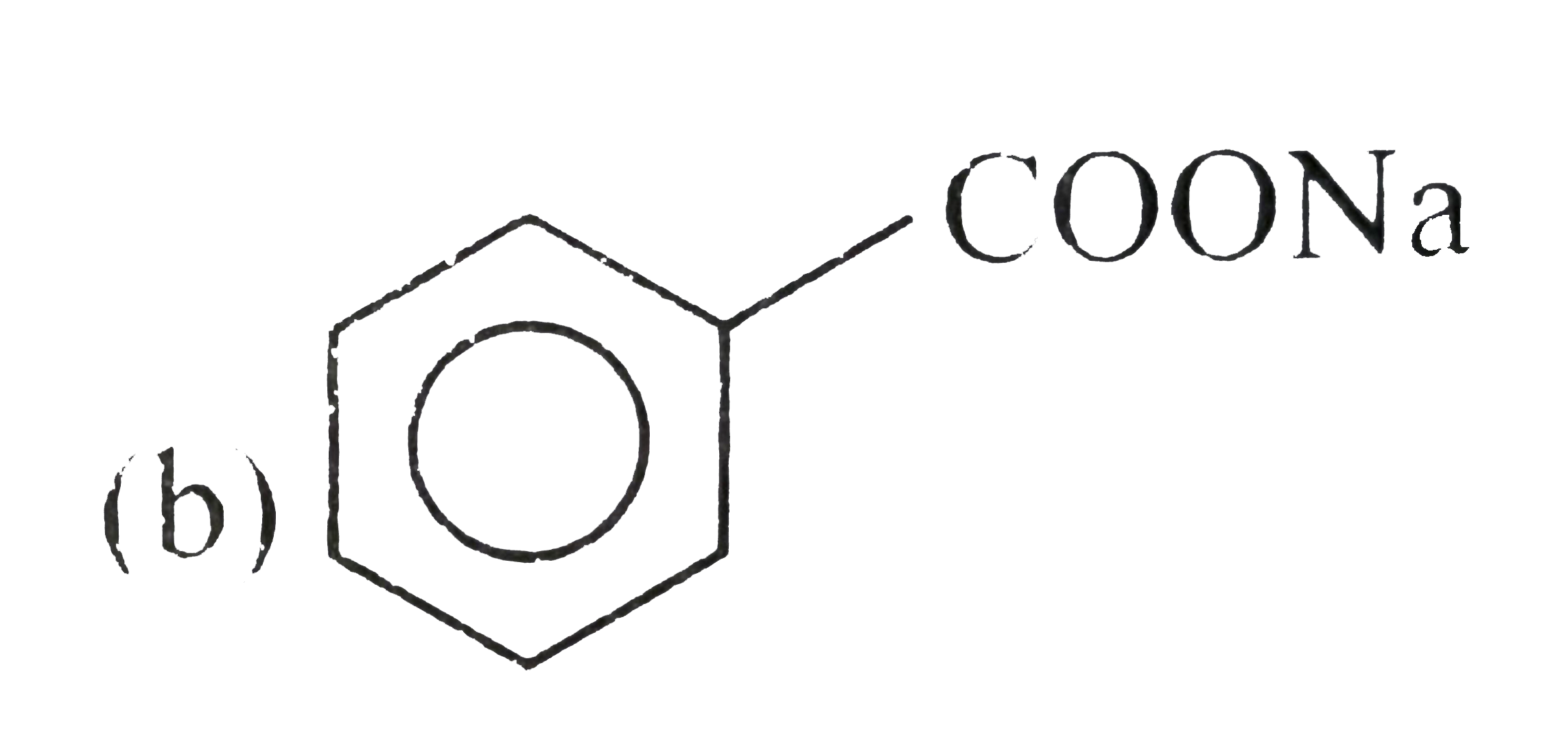 .
.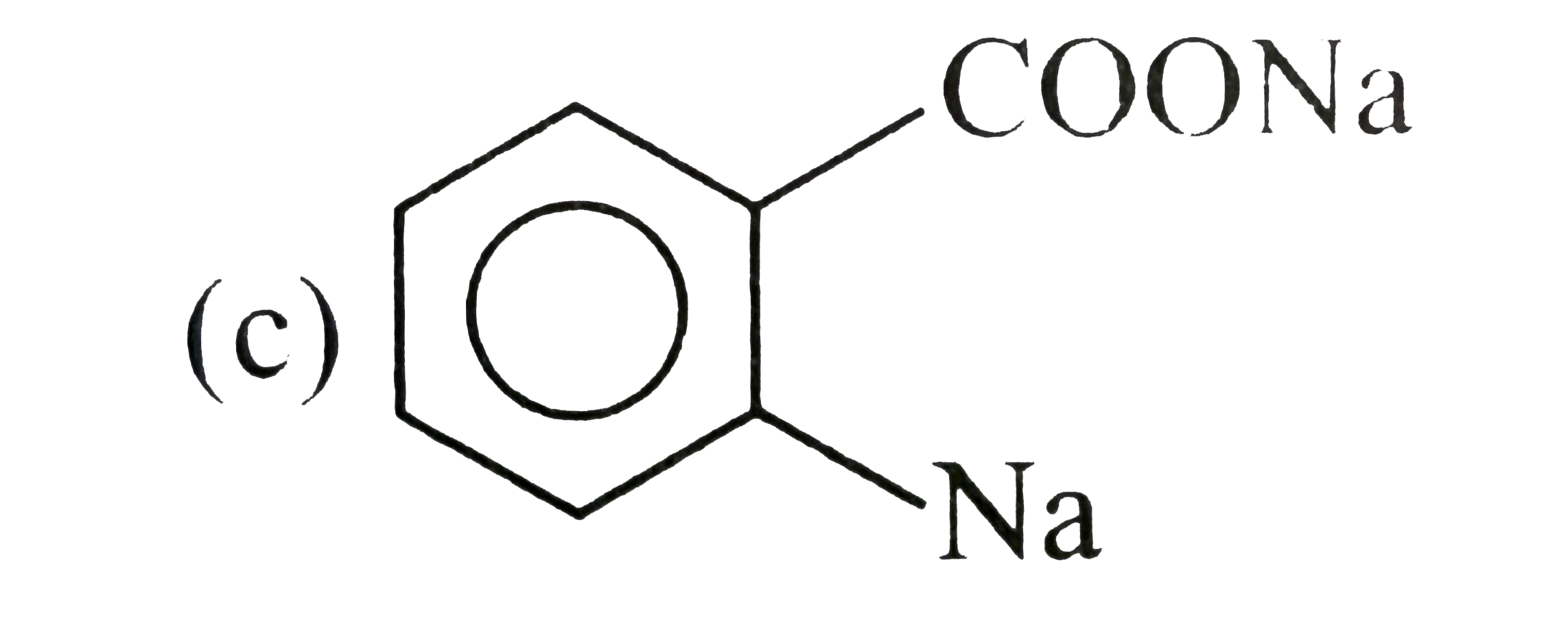 .
.