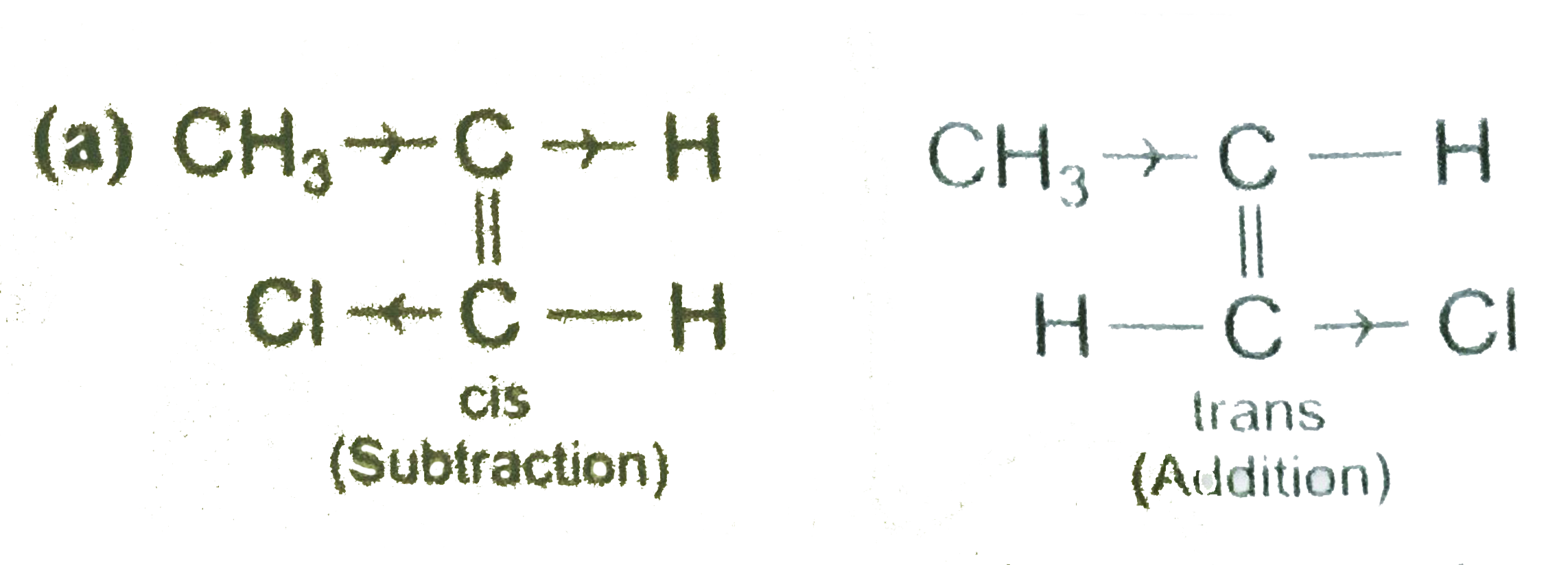A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-ORGANIC COMPOUNDS CONTAINING HALOGENS-Assertion-Reasoning Questions
- Assertion: Bromobenzene upon reaction with Br(2)//Fe gives 1,4-dibromo...
Text Solution
|
- Assertion: C Cl(4)and H(2)O are immiscible . Reason : C Cl(4) is a p...
Text Solution
|
- Assertion: Styrence on reaction with HBr gives 1-bromo-1-phenylethane ...
Text Solution
|
- Assertion: Aryl undergoes nucleophilic substitution with ease Reaso...
Text Solution
|
- Assertion: CHI(3) gives a precipitate with AgNO(3) solution on heating...
Text Solution
|
- Assertion: The reactivity order for S(N)1 reation is Ar(3)CXgtAr(2)CHX...
Text Solution
|
- Assertion: Trans-2-chloro propene has higher dipole moment than cis-2-...
Text Solution
|
- Assertion (A): Iodine is more soluble in C Cl(4) than in water. Reas...
Text Solution
|
- Chlorination of allylic hydrogen is difficult than vinylic hydrogen. ...
Text Solution
|