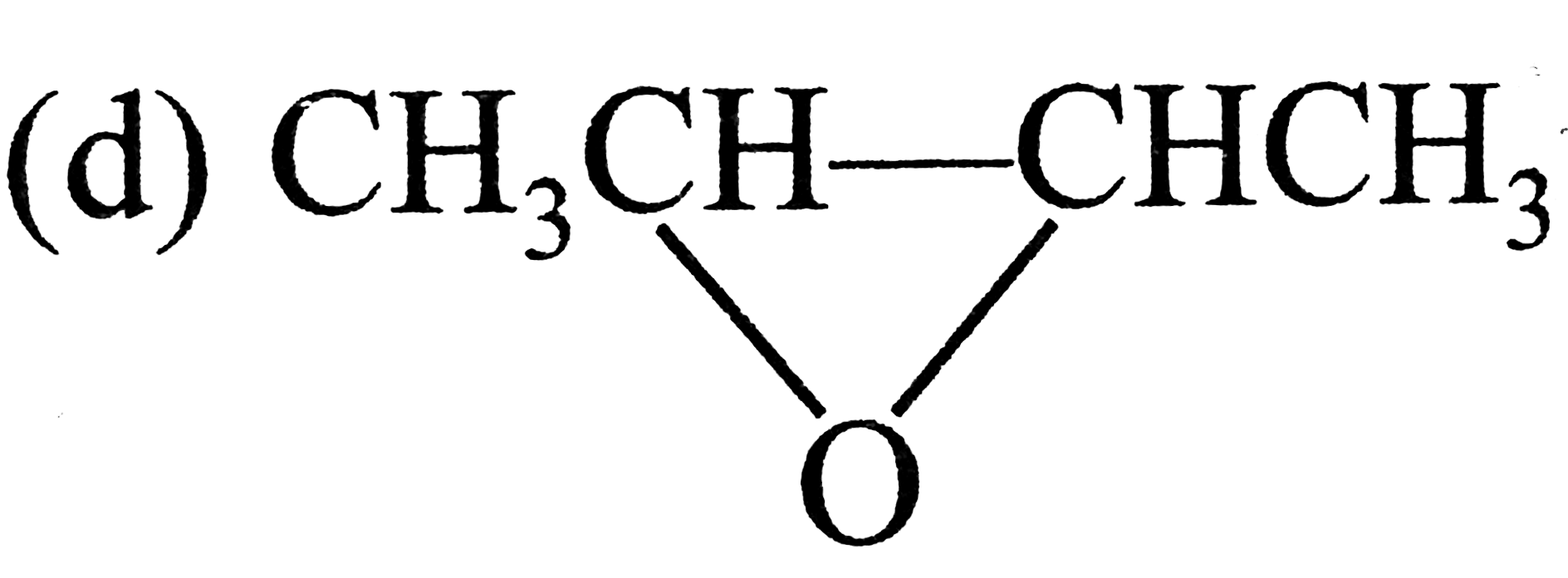
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-ALCOHOLS, PHENOLS AND ETHERS-Section D - Chapter End Test
- Which of the following compounds on reaction with CH(3)MgBr will give ...
Text Solution
|
- A and B are:
Text Solution
|
- Dipole moment of CH(3)CH(2)CH(3)(I),CH(3)CH(2)OH(II) and CH(3)CH(2)F(I...
Text Solution
|
- Acidic nature is more for
Text Solution
|
- Phenol can be distinguished from aliphatic alcohol with
Text Solution
|
- The strongest acid among the following is
Text Solution
|
- Phenol is less acidic than
Text Solution
|
- B(mix)overset(conc.HI)underset(2) larr CH(3))(2)C-O-CH(3) overset("anh...
Text Solution
|
- The boiling points to isomertic alcohols follow the order
Text Solution
|
- Which of the following is a parimary alcohol?
Text Solution
|
- Complete the following reaction
Text Solution
|
- The compound that reacts faster with Lucas reagent (conc.HCl +ZnCl(2))...
Text Solution
|
- The-OH group of Methyl alcohol cannot be replaced by chlorine by the t...
Text Solution
|
- The calcohol which easily reacts with conc. HCI is
Text Solution
|
- Because of resonance the oxygen atom of -OH group of phenol
Text Solution
|
- The compound obtained by heating salicylic acid with phenol in the pre...
Text Solution
|
- When phenol is allowed to react with Br(2) in (i) CS(2) solution and (...
Text Solution
|
- When rectified spirit and benzene are distilled togther, the first fra...
Text Solution
|
- Alcohols react with Grignard reagent to form
Text Solution
|
- The boiling point of methanol is greater than that of Methyl thiol bec...
Text Solution
|
- Which of the following will not form a yellow precipitate on heating w...
Text Solution
|