A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
A2Z|Exercise Ethers|34 VideosALCOHOLS, PHENOLS AND ETHERS
A2Z|Exercise Section B - Assertion Reasoning|20 VideosALCOHOLS, PHENOLS AND ETHERS
A2Z|Exercise Chemical Properties Of Alcohols|61 VideosALDEHYDES, KETONES AND CARBOXYLIC ACID
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ALCOHOLS, PHENOLS AND ETHERS-Phenols
- 'A' and 'B' respectively are
Text Solution
|
- The major product [P] is
Text Solution
|
- In the following compounds, (I) (II) (III) (IV) the decreasing...
Text Solution
|
- Which of the following reagents can be used to separate a mixture of p...
Text Solution
|
- The compound with the lowest boiling point, that is, the most valatil...
Text Solution
|
- Phenol can be distinguished from aliphatic alcohol with
Text Solution
|
- Cresols are
Text Solution
|
- Which of following is phenolic?
Text Solution
|
- On heating aqueous solution of benzene diazonium chloride, which is fo...
Text Solution
|
- Phenolphthalein is obtained by heating phtahalic anhydride with conc. ...
Text Solution
|
- Reaction of phenol with dil. HNO(3) gives
Text Solution
|
- Phenol is less acidic than
Text Solution
|
- Sodium pheoxide reacts with CO(2) at 400K and 4-7 atm pressure to give
Text Solution
|
- A compound that easily undergoes bromination is
Text Solution
|
- For pheonl, which of the following statements is correct
Text Solution
|
- When heated with NH(3) under pressure alone or in presence of zince ch...
Text Solution
|
- At low temperature phenol reacts wity Br(2) in CS(2) to form
Text Solution
|
- The compound obtained by heating salicylic acid with phenol in the pre...
Text Solution
|
- What amount of bromine will be required to convert 2g of phenol into 2...
Text Solution
|
- Phenol reacts with C CI(4) is presence of aqueneous alkali and forms a...
Text Solution
|
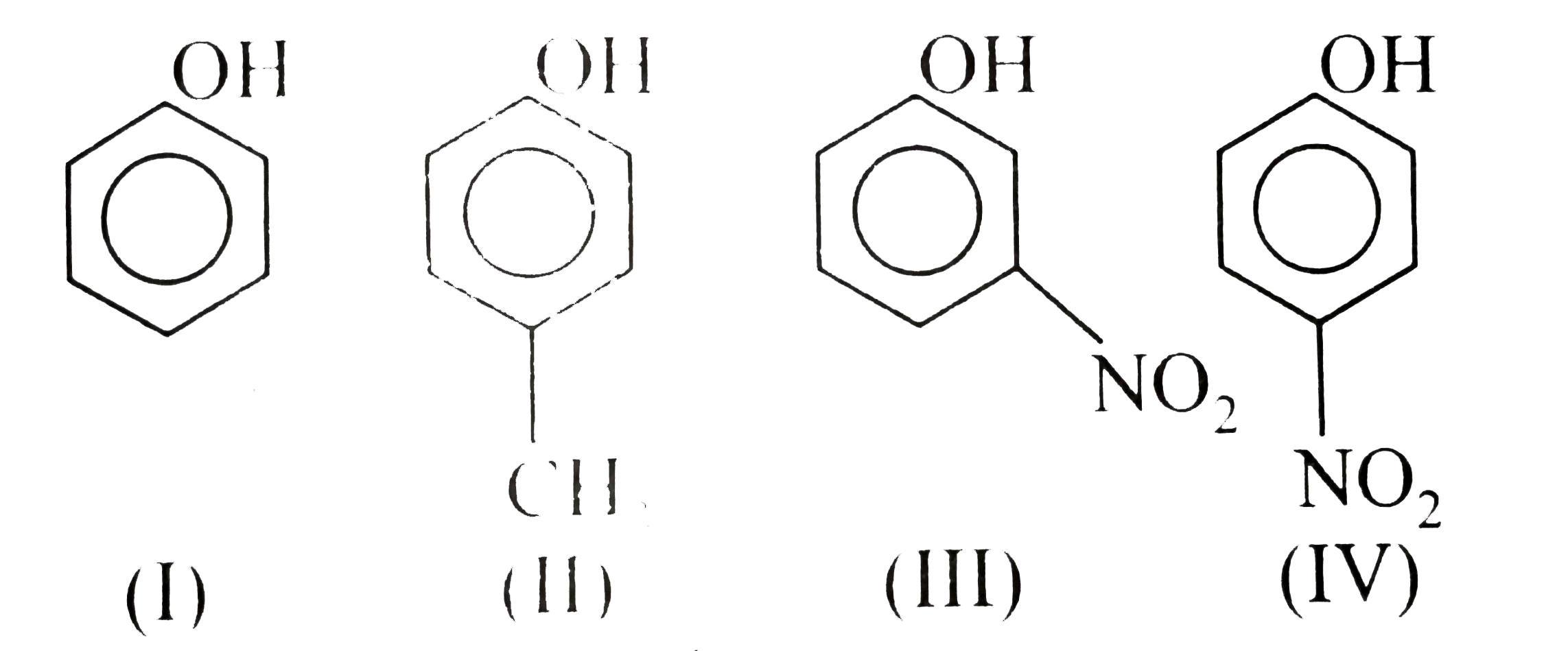 (II)
(II)  (III)
(III)  (IV)
(IV) 