A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-ALCOHOLS, PHENOLS AND ETHERS-Section B - Assertion Reasoning
- Assertion: Solubility of n-alcohol in water decreases with increases i...
Text Solution
|
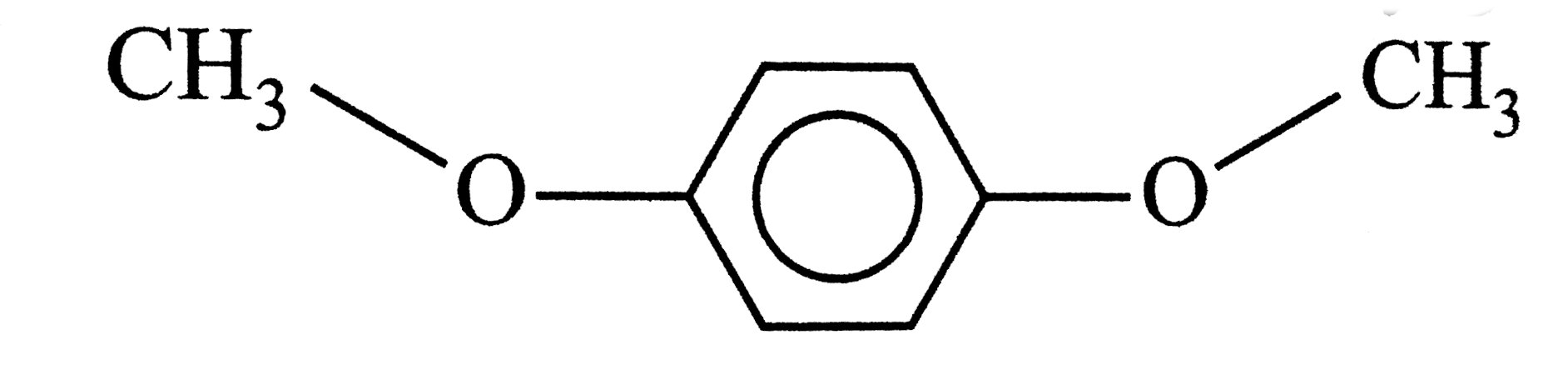
- Assertion: p-dimethoxybenzene is polar. Reason: The different oriena...
Text Solution
|
- Assertion: C(2)H(5)O is more reactive than C(2)H(5)CI. Reason: I bei...
Text Solution
|
- Assertion: Ethanol is more viscous than glycerol. Reason: Both posse...
Text Solution
|
- Asserion: A small amount of ethanol is added to CHCI(3) bottles. Rea...
Text Solution
|
- Assertion: Alcohols cannot be dried by CaCI(2). Reason: CaCI(2) form...
Text Solution
|
- Asseriton: Methanol is stronger acid than water. Reason: All alcohol...
Text Solution
|
- Assertion: Acid cataysed dehydration of t-butanol is faster than n-but...
Text Solution
|
- Assertion: Alcohols give only substitution products with HX and not el...
Text Solution
|
- Assertion: p-nitrophenol is a stronger acid than o-nitrophenol. Reas...
Text Solution
|
- Assertion: o-and p-nitrophenol can be separated by steam distillation....
Text Solution
|
- Assertion: Alcohol and ohenol can be distinguished by sodium hydroxide...
Text Solution
|
- Assertion: Isobutanal does not give iodoform test. Reason : It does ...
Text Solution
|
- Assertion: m-Methoxyphenol is a stronger acid than p-methoxyphenol. ...
Text Solution
|
- Assertion: 2-Phentanol and 3-pentanol cannot be distinguished by iodof...
Text Solution
|
- Assertion: equimolar mixture of conc. HCI and anhydrous zinc chloride ...
Text Solution
|
- Assertion: Anisole undergoes eletctrophilic substitution at o-and p-po...
Text Solution
|
- Assertion: Alcohols have higher boiling points than ethers of comparab...
Text Solution
|
- Assertion. t-Buty1 Methyl ether is not prepared by rthe reaction of t-...
Text Solution
|
- Assertion: Benzenediazonium chloride on boiling with water gives pheno...
Text Solution
|