A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
MOCK TEST
A2Z|Exercise Single correct Answer type question|40 VideosMOCK TEST
A2Z|Exercise Assertion-Reasoning|5 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF METALS
A2Z|Exercise Section D - Chapter End Test|30 VideosORGANIC COMPOUNDS CONTAINING HALOGENS
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-MOCK TEST-Mock Test 2
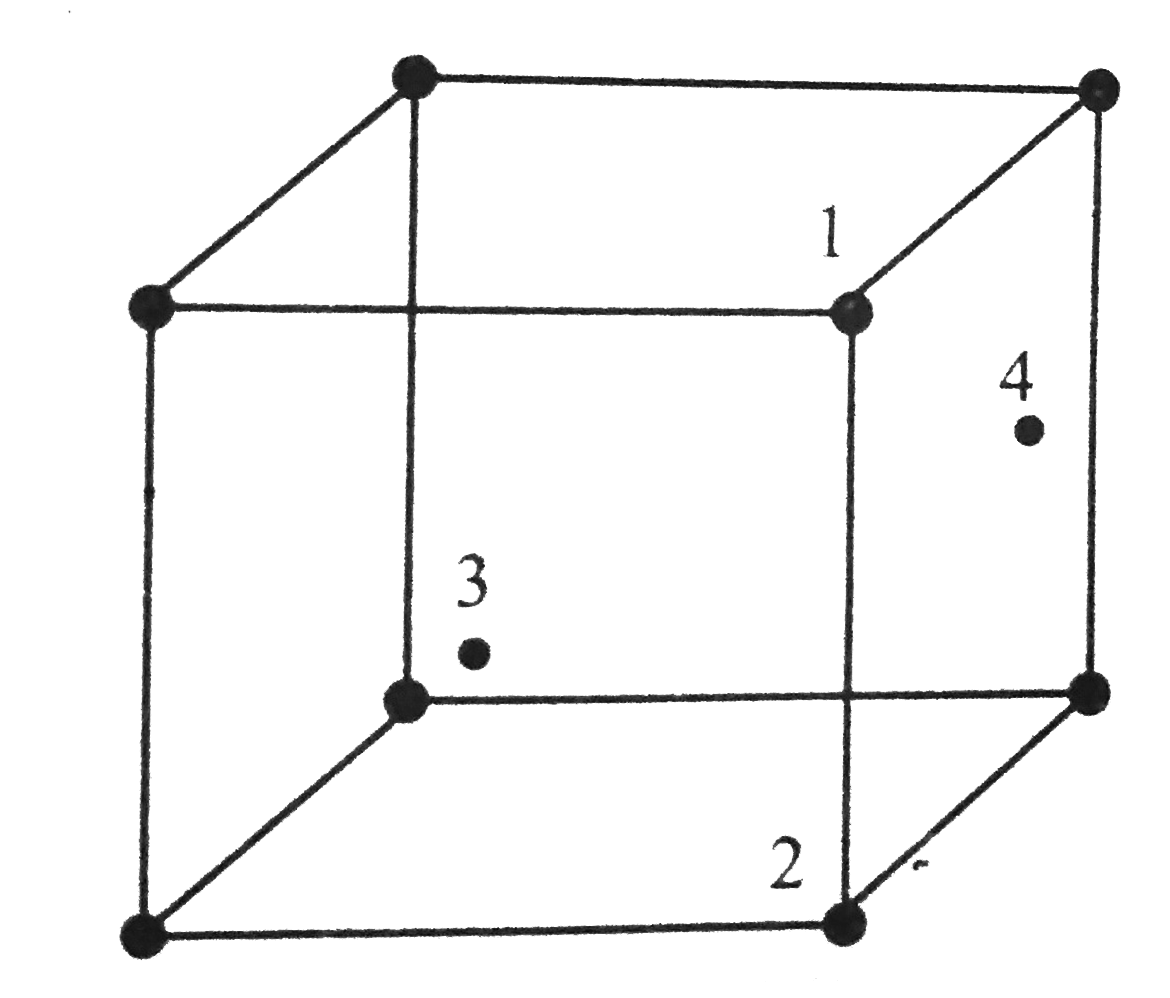
- In an fcc unit cell, atoms are numbered as shown below The atoms ...
Text Solution
|
- What is the density of Na(2)O having antifluorite-type crystgal stryct...
Text Solution
|
- In the calcum fluaride structure, the coordination bumber of the catio...
Text Solution
|
- Assuming each salt to be 90% dissociated which of the following will h...
Text Solution
|
- When a solution is separated from a solvent by a semi-permeable membra...
Text Solution
|
- The values of wedge(m)^(oo) forNH(4)Cl,NaOH, and NaCl are, respectivel...
Text Solution
|
- 0.5F of electricity is passed through 500mL of copper sulphate solutio...
Text Solution
|
- Rate constant of a reaction with a virsus is 3.1 xx 10^(-4) s^(-1). Ti...
Text Solution
|
- 2N2O5 rarr 4 NO2 +O2 If -(D[N2O5])/(dt) =k1[N2O5] (d[NO2])/(dt) =k...
Text Solution
|
- Of which of the following colloidal systems, fog is an example?
Text Solution
|
- Soaking of water by a sponge is an example of
Text Solution
|
- A radioisotope has half life of 10 years. What percentage of the origi...
Text Solution
|
- When .(92)U^(238) decauys it emits an a-particle. The new nuclide in t...
Text Solution
|
- A will ……..by E1 reaction .
Text Solution
|
- Major product of this reaction is .
Text Solution
|
- CH3CH2CHO overset (NaOH, Delta) underset((aldol)) (rarr)A,A is.
Text Solution
|
- An optically active compound X has molecular formular C4H8 O3. It e...
Text Solution
|
- What is the end product of following reaction . overset ((i) H...
Text Solution
|
- . overset (HNO2) (rarr) A (Major product ), A is .
Text Solution
|
- Which A gives red colour in the reaction A overset ((i) HNO2)unders...
Text Solution
|
- Of the follwing , the most acidic is .
Text Solution
|