Similar Questions
Explore conceptually related problems
Recommended Questions
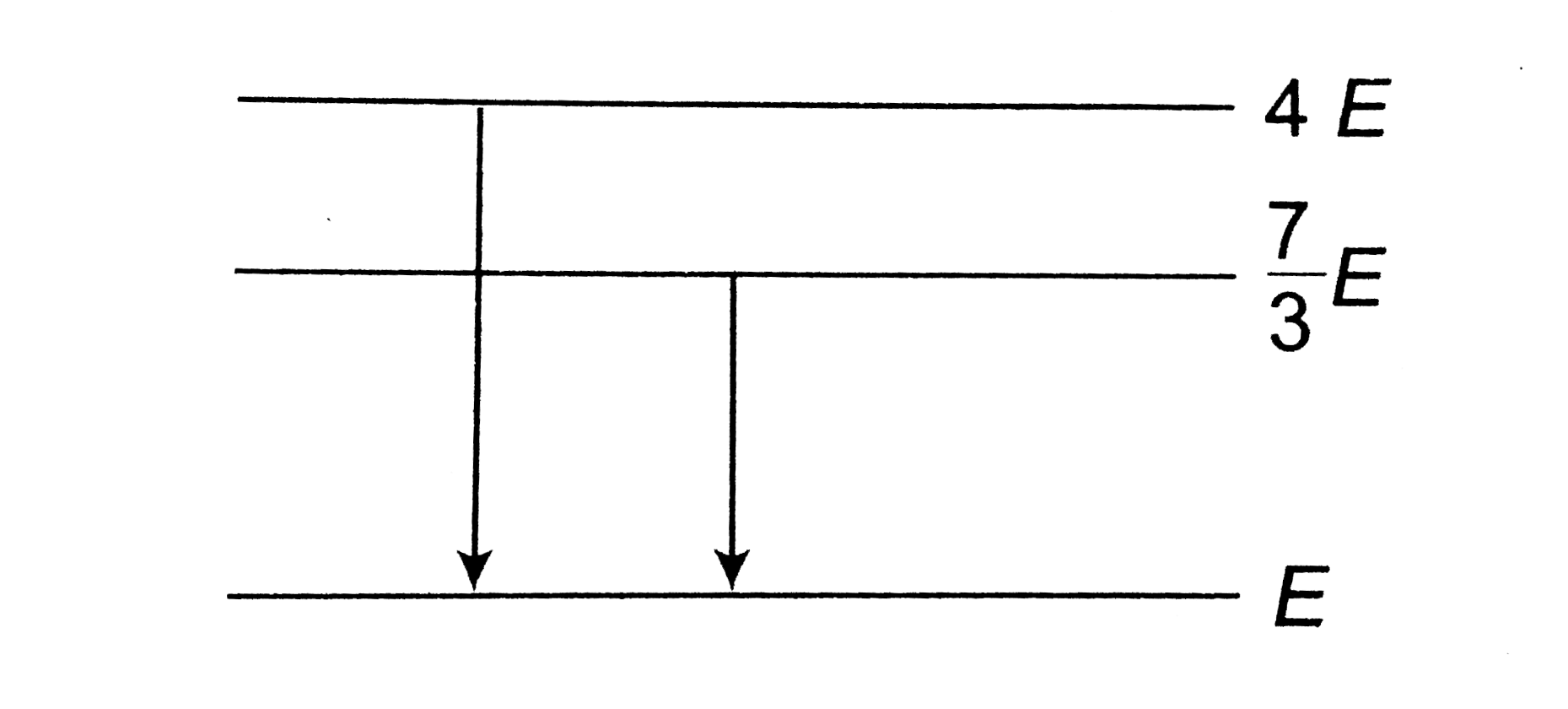
- The following diagram indicates the energy levels of a certain atom wh...
Text Solution
|
- In a set of experiment on a hydrogen on a hypotherical one-electron at...
Text Solution
|
- The follwing diagram indicates the energy levels of a certain atom whe...
Text Solution
|
- The following diagram indicates the energy levels of a certain atom wh...
Text Solution
|
- A proton with KE equal to that a photono (E = 100 keV). lambda(1) is t...
Text Solution
|
- सलग्न चित्र में किसी का ऊर्जा -स्तर प्रदर्शित है । जब परमाणु 2E से E ...
Text Solution
|
- एक प्रोटॉनकी गतिज ऊर्जा एक फोटॉन की ऊर्जा E के बराबर है । यदि प्रो...
Text Solution
|
- The following figure shows the energy levels of a particular atom. Whe...
Text Solution
|
- The given diagram indicates the energy levels of a certain atom. When ...
Text Solution
|