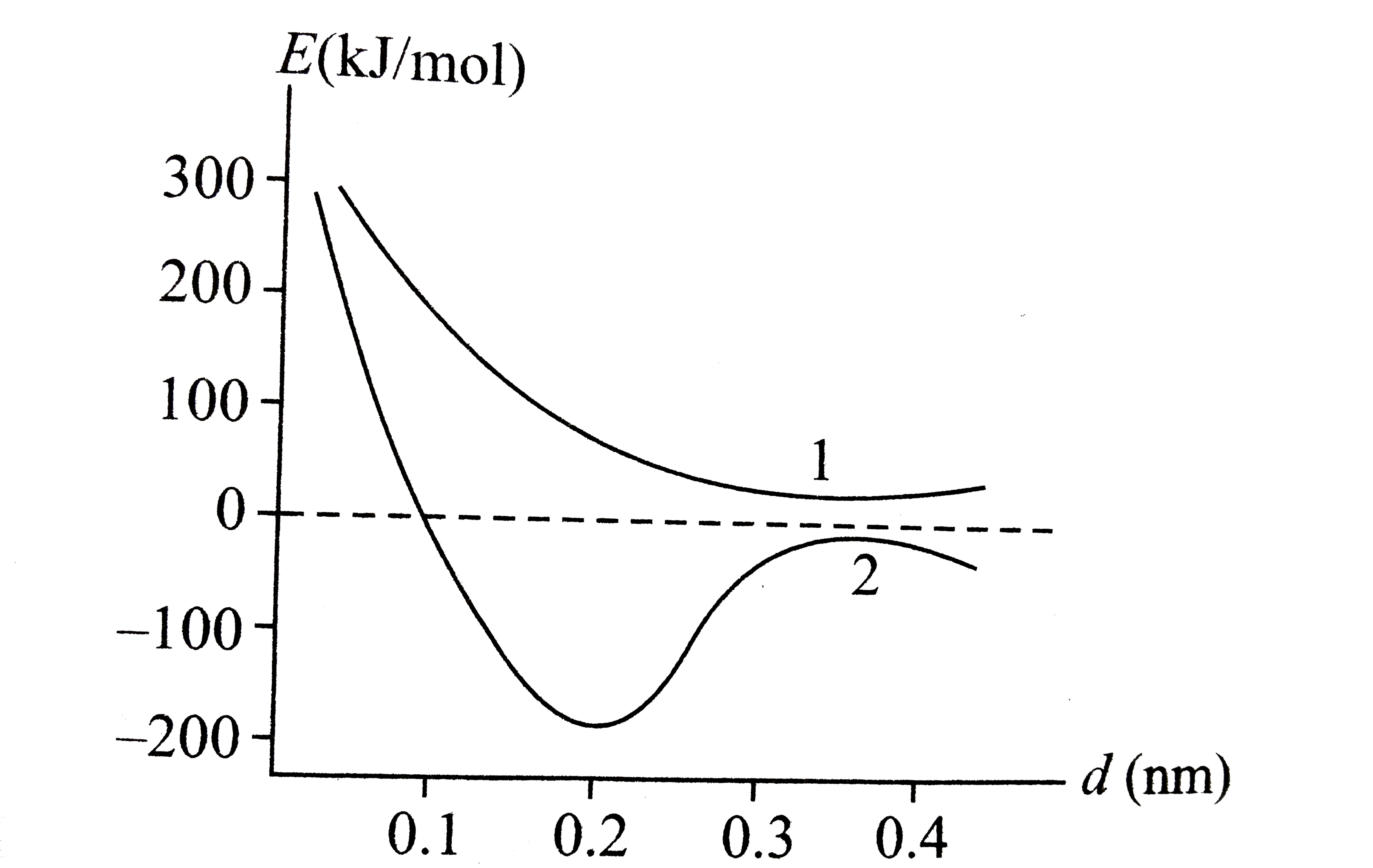
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Force Of Attraction|22 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Bond Enthalpy, Bond Angle And Bond Length|23 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Vsepr Theory And Hybridisation|46 VideosATOMIC STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 VideosCHEMICAL EQUILIBRIUM
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Molecular Orbitial Theory
- Which one of the following constitutes a group of the isoelectronic sp...
Text Solution
|
- In the of the following pairs of molecules /ions both the species are ...
Text Solution
|
- Consider the given figure showing the formation of H(2)^(+) ion depen...
Text Solution
|
- Which of the following is paramagentic?
Text Solution
|
- N(2) and O(2) are converted into monocations, N(2)^(+) and O(2)^(+) r...
Text Solution
|
- Which of the following order is correct for the bond dissociation ene...
Text Solution
|
- Which of the following statement is incorrect?
Text Solution
|
- Which of the following statement is incorrect?
Text Solution
|
- S(1): The HOMO in F(2) is pi* 2p(s) = pi*2p, molecular orbitals S(2)...
Text Solution
|
- The nodal plane is the pi -bond of ethene is located in :
Text Solution
|
- Among the following , the paramagnetic compound is :
Text Solution
|
- Which of the following option with respect to increasing bond dissocia...
Text Solution
|
- Write the molecular orbital electron distribution of oxygen (O(2)) Spe...
Text Solution
|
- The cyanide ion CN and N(2) are isoelectronic, but in contrast to CN^(...
Text Solution
|
- Which of the following comnpounds is paramagnetic?
Text Solution
|
- The number of antibonding electron pairs in O(2)^(2-) molecular ion on...
Text Solution
|
- The correct order of decreasing C-O bond length of (1) CO,(II)CO(3)^(2...
Text Solution
|
- The bond length the species O(2), O(2)^(+) and O(2)^(-) are in the ord...
Text Solution
|
- In a matallic crystal the .
Text Solution
|
- The common features among the species CN^- , CO and NO^+ are :
Text Solution
|