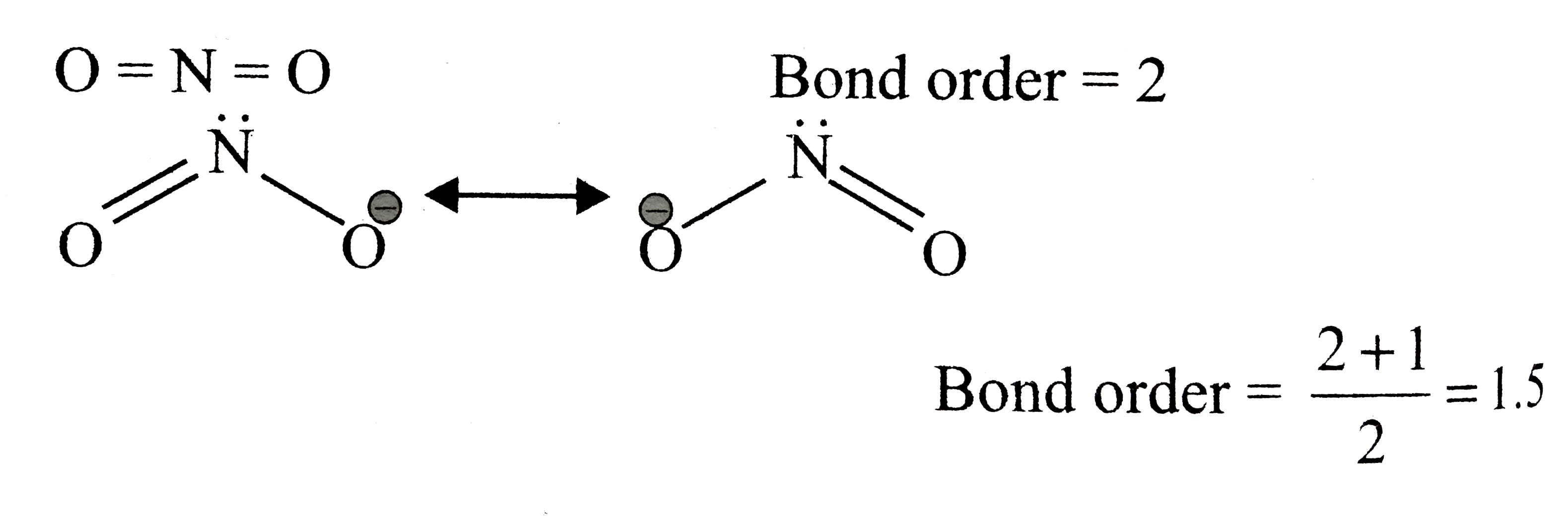
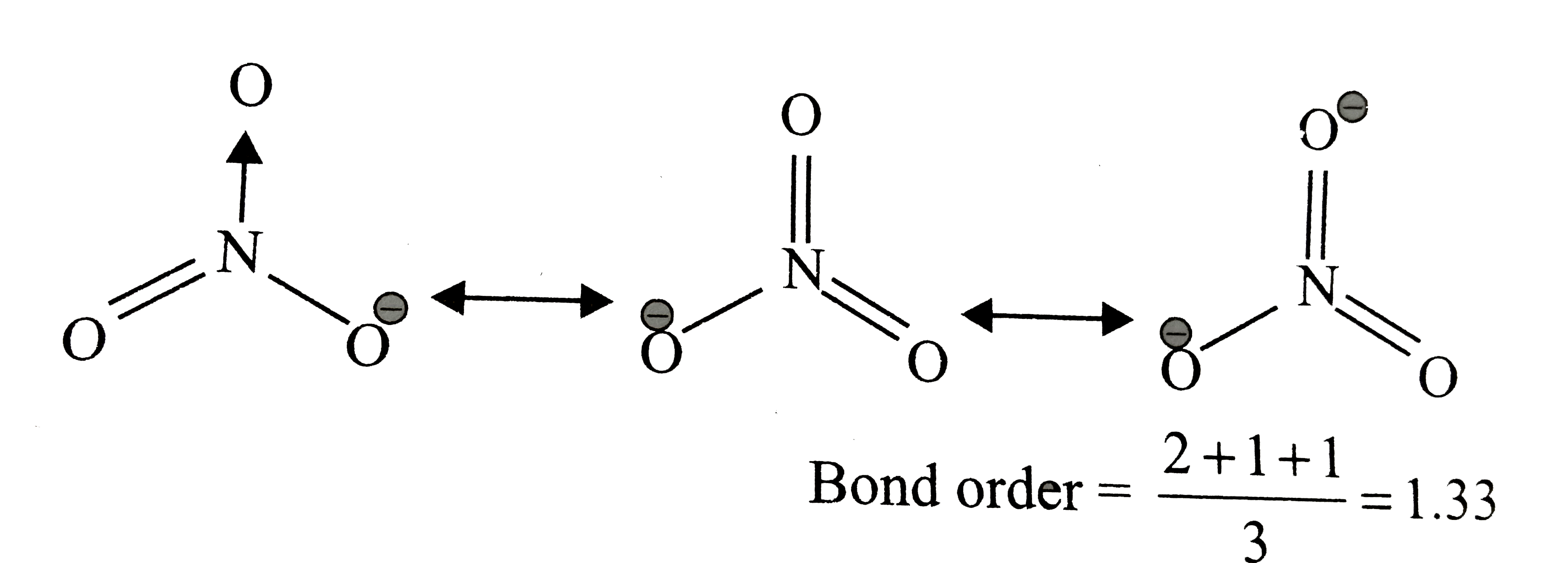
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Section B - Assertion Reasoning|37 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise AIPMT/ NEET Questions|89 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Force Of Attraction|22 VideosATOMIC STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 VideosCHEMICAL EQUILIBRIUM
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Bond Enthalpy, Bond Angle And Bond Length
- The bond energies in NO, NO^(+), NO^(-) follow the order
Text Solution
|
- The correct order of O - O bond length in O(2),H(2)O(2) and O(3) is
Text Solution
|
- Among the species CO(2),CH(3)COO',CO,CO(3)^(2-)HCHO which has the veak...
Text Solution
|
- In the series ethane, ethylene and acetylene the C-H bond energy is
Text Solution
|
- As the p - charcter increases the bond angle in in hydrid orbital form...
Text Solution
|
- Which of the least bond angle ?
Text Solution
|
- Which of the following sequence represents the correct increasing orde...
Text Solution
|
- The correect order of decreasing bond angle is
Text Solution
|
- In which of the following compound all the bond angles are same
Text Solution
|
- Consider the following molecules : H(2)O H(2)S H(2) Se H(2)Te I ...
Text Solution
|
- Consider the following statement(s) CH(3) = X and CV(3) = V (i)Which...
Text Solution
|
- Percentage of p- character in each orbital of centain atom used bondi...
Text Solution
|
- The ONO angle is maximum in :
Text Solution
|
- Arrange the following in order of decreasing N - O bond length NO(2)^(...
Text Solution
|
- The highest amount of s-character is observed in :
Text Solution
|
- In which of the following pairs , bond angle is 109^@28' ?
Text Solution
|
- Which among the following has smallest bond angle ?
Text Solution
|
- The correct order of bond strength is
Text Solution
|
- The decreasing values of bond angles from NH3 (106^@) to SbH3 ( 101^@...
Text Solution
|
- The molecule having smaller bond angle is
Text Solution
|