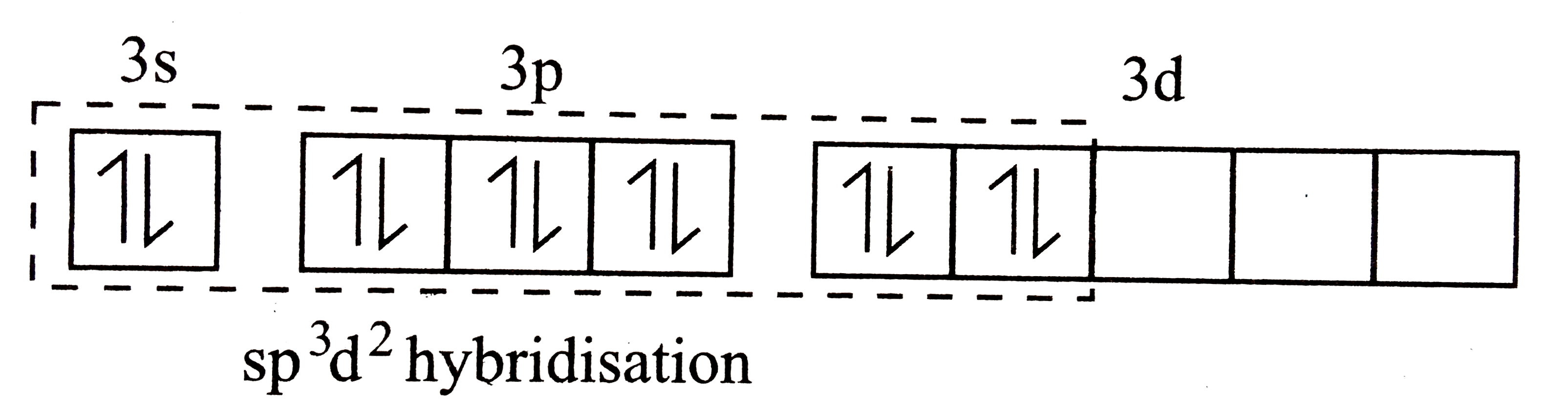
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise AIPMT/ NEET Questions|89 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise AIIMS Questions|31 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Bond Enthalpy, Bond Angle And Bond Length|23 VideosATOMIC STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 VideosCHEMICAL EQUILIBRIUM
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Section B - Assertion Reasoning
- Statement : p-dimethoxy benzene is polar molecule . Explanation : T...
Text Solution
|
- Statement : The lattice energy of silver halids is AgF gt AgCl gt AgB...
Text Solution
|
- Assertion : In CH(3)NCO, the angles C - N - C and N - C - O are not id...
Text Solution
|
- Assertion : In IOF(4)^(-) a single lone pair is present an iodine atom...
Text Solution
|
- Assertion : Molecular having different hybridisation can have same sha...
Text Solution
|
- Assertion :SO(2),NO(3)^(-) and CO(3)^(2-) are isoelectronic as well ...
Text Solution
|
- Assertion : Carbon has unique ability to form p pi-p pi multiple bonds...
Text Solution
|
- Assertion : F bond angle p is equal to the bond angle Q but not preci...
Text Solution
|
- Assertion : Elemental nitrogen exist as a diatomic molecule and phospo...
Text Solution
|
- Assertion : Amongst the oxo acids of halogens, HOCI, HOBr and HOI , th...
Text Solution
|
- Assertion : Aluminium chloride in acidified aqueous solution from octa...
Text Solution
|
- Assertion :A molecule of Buckminsterfullerene exhibita aromatic charac...
Text Solution
|
- Assertion : The double bond in C(2) molecule consider of both pi bon...
Text Solution
|
- Assertion : To obtain effefctive p pi-p pi overlap, the size of the de...
Text Solution
|
- Assertion :dimenthyl ether and disilyl ether both readily form complex...
Text Solution
|
- Assertion : Solubility of Lil is more than that of LiBr. Reason :Li...
Text Solution
|
- Assertion : AI^(3+) forms more ionic compound in comparison to Ga^(3+...
Text Solution
|
- Assertion : NF(3) has tendency to act as a donor molecule. Reason : ...
Text Solution
|
- Assertion : Ortho boric acid crystal are hard and cannot be broken ea...
Text Solution
|
- Assertion :The orystal sturctures of NaHCO(3) and KHCO(3) both show in...
Text Solution
|