A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise AIIMS Questions|31 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Assertion - Reasoning Questions|6 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Section B - Assertion Reasoning|37 VideosATOMIC STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 VideosCHEMICAL EQUILIBRIUM
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL BONDING AND MOLECULAR STRUCTURE-AIPMT/ NEET Questions
- Which of the following is the electron-deficient molecule?
Text Solution
|
- Which one of the following arrangements represents the correct order o...
Text Solution
|
- Which molecule has trigonal planar geometry ?
Text Solution
|
- The molecule having permanent dipole moment is
Text Solution
|
- Which is expected to show paramagnetism ?
Text Solution
|
- The electronegaivity difference between N and F is greater than that b...
Text Solution
|
- In which of the following molecules all the bonds are not equal ?
Text Solution
|
- The correct order of electronegativity regarding the hybrid orbitals o...
Text Solution
|
- Which of the following species has a linear shape ?
Text Solution
|
- Which of the following is not isostructural with SiCI(4) ?
Text Solution
|
- Which of the following is not a correct statement ?
Text Solution
|
- Which one of the following orders is not correct in accordance with t...
Text Solution
|
- The number of unpaired electrons in a parmamagnetic diatomic molecule ...
Text Solution
|
- Which of the following pair are isotructural ?
Text Solution
|
- The element having lowest ionisation energy among the following is
Text Solution
|
- The correct order of decreasing C-O bond length of (1) CO,(II)CO(3)^(2...
Text Solution
|
- Which of the following posses maximum hydration energy ?
Text Solution
|
- The angular shape of none molecule (O(3)) consists of
Text Solution
|
- Decreasing order of bond angle of (NO(2)^(o+), NO(2), NO(2)^(ɵ) is
Text Solution
|
- Four diatomic species are listed in different sequence .Which of thes...
Text Solution
|
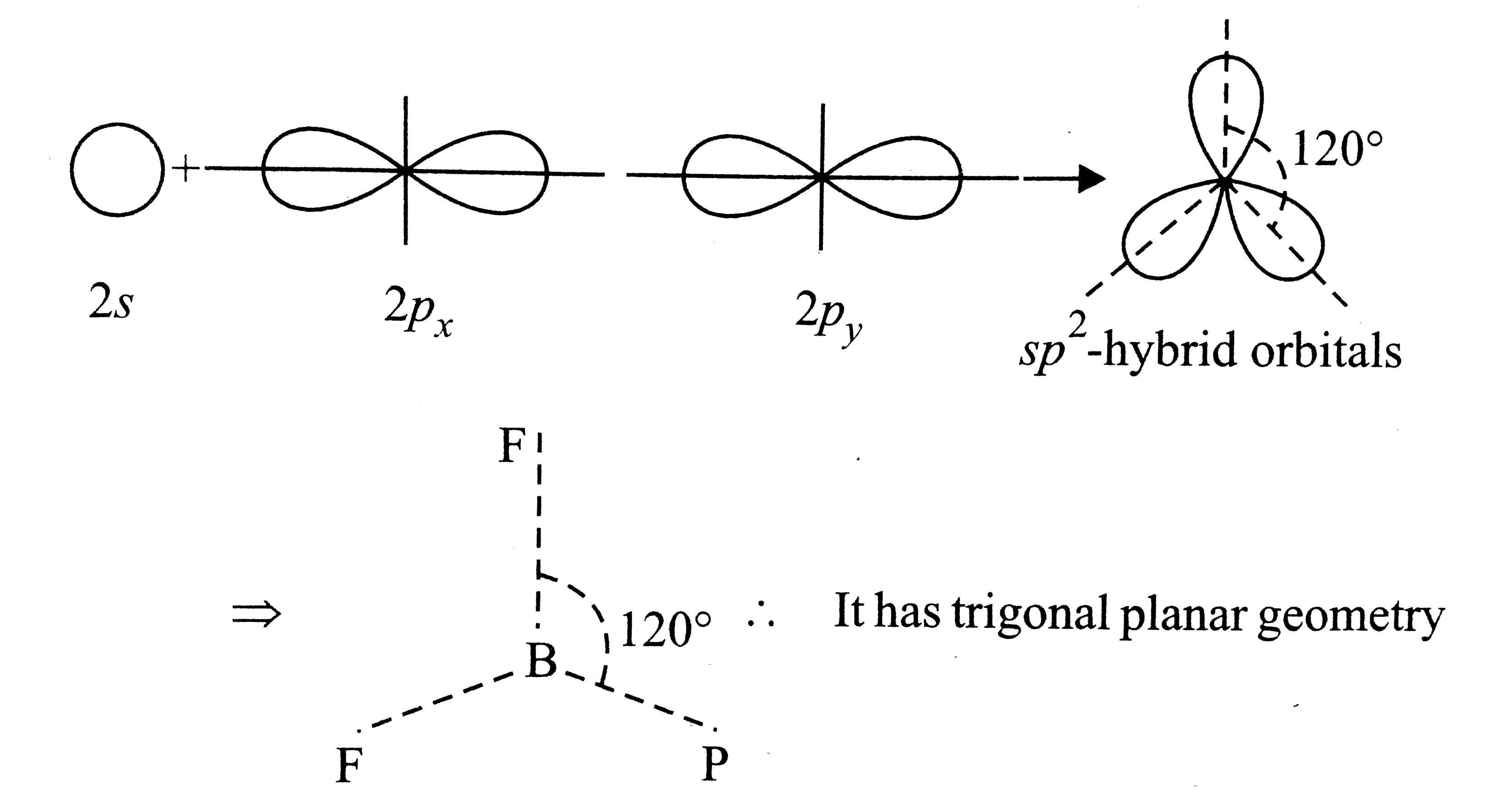 .
.