A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Assertion - Reasoning Questions|6 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise AIPMT/ NEET Questions|89 VideosATOMIC STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 VideosCHEMICAL EQUILIBRIUM
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL BONDING AND MOLECULAR STRUCTURE-AIIMS Questions
- If the electron pair forming a bond between two atoms and B is not in ...
Text Solution
|
- Which of the following is a polar compound ?
Text Solution
|
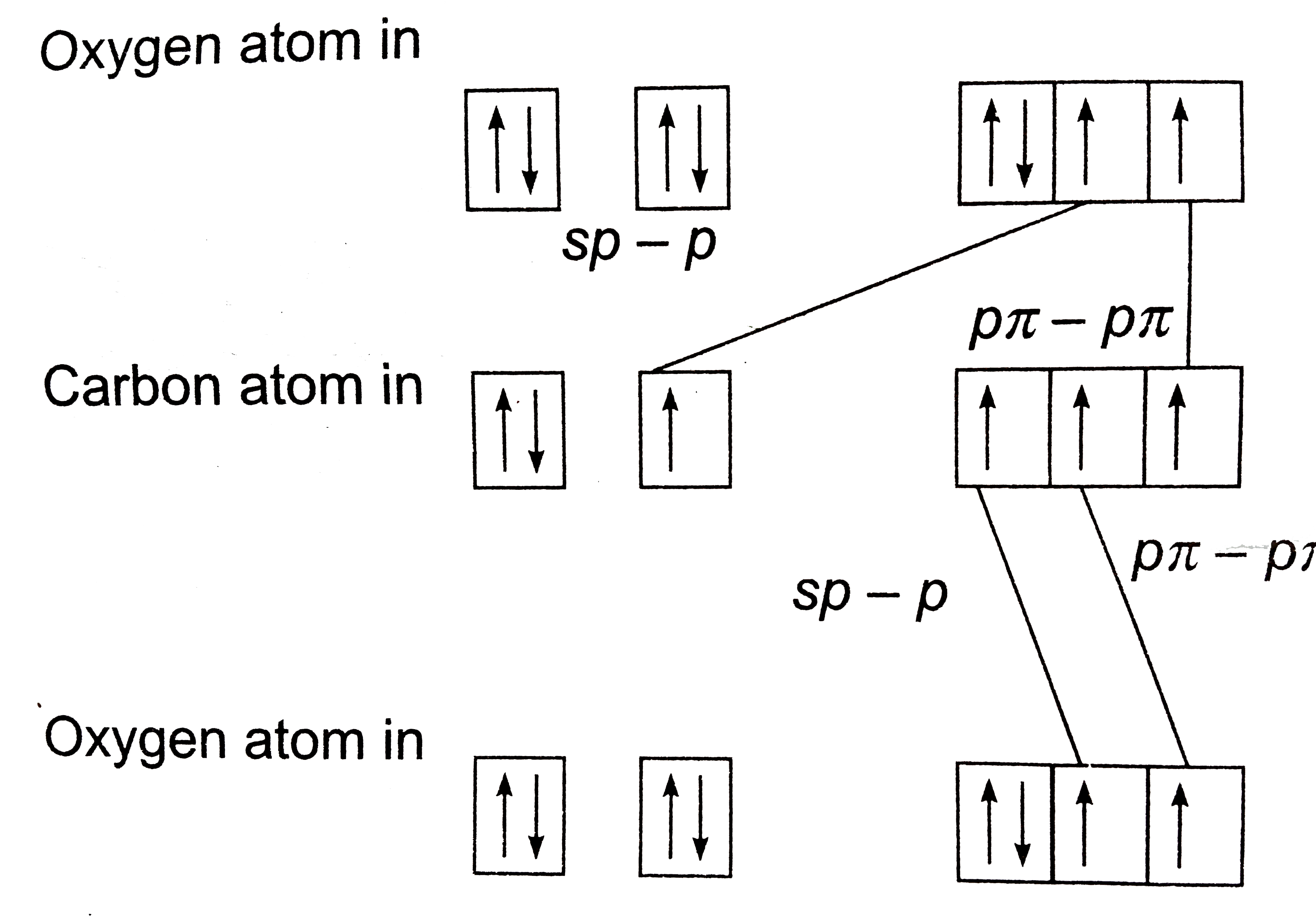
- In which of the following there exists a p pi-p pi bonding
Text Solution
|
- Which of the following statement is not correct ?
Text Solution
|
- Noble gases have compleately filled valance shall i.e. m^(2)sp^(2) exc...
Text Solution
|
- The molecule of CO(2) has 180^(@) bond angle it one be explained on th...
Text Solution
|
- Which of the following compounds the one having linear structure is
Text Solution
|
- The isoelectronic pair is
Text Solution
|
- Bond order of O(2), O(2)^(-) and O(2)^(2-) is in order
Text Solution
|
- Which of the following does not exist on the basis of molecule orbital...
Text Solution
|
- Which of the following species have maximum number of unpaired elect...
Text Solution
|
- Give reason in one or two sentences form the following: 'o-nitrophenol...
Text Solution
|
- Water has high heat of vaporisation due to ?
Text Solution
|
- Why is ice less denser than water and what kind of attractive force mu...
Text Solution
|
- Ethyl alcohol (C(2)H(5)OH) has higher boiling point than dimethyl ethe...
Text Solution
|
- Which one is the highest melting halide ?
Text Solution
|
- In the formation of a molecule by an atom ?
Text Solution
|
- Which of the following exhibits the weakest intermolecular forces?
Text Solution
|
- H(2)O is depolar, wheras BeF(2) is not. it because
Text Solution
|
- Which is incorrect regarding S and P mixing (along Z axis.) ?
Text Solution
|