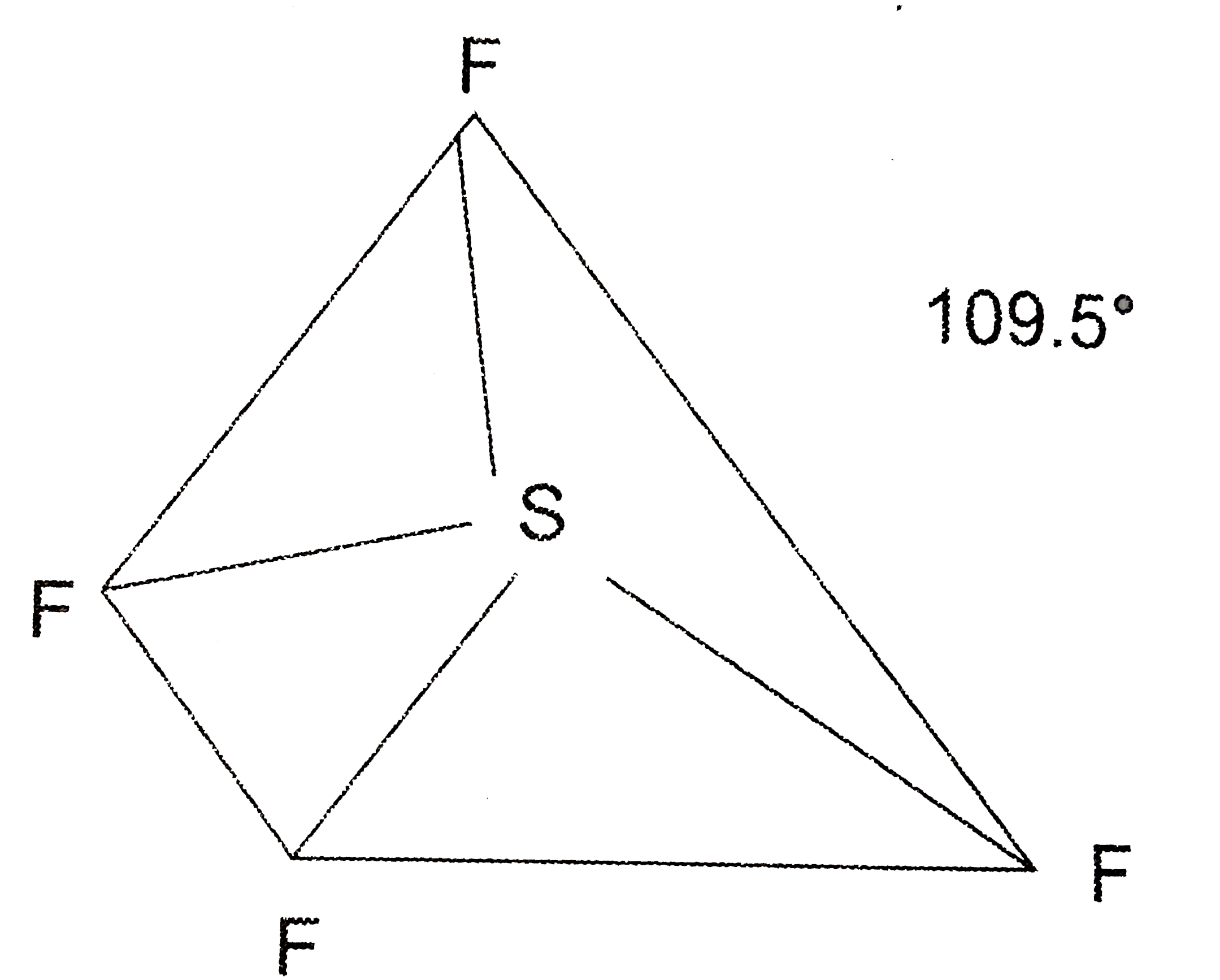
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
A2Z|Exercise AIIMS Questions|31 VideosATOMIC STRUCTURE
A2Z|Exercise Section D - Chapter End Test|30 VideosCHEMICAL EQUILIBRIUM
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Assertion - Reasoning Questions
- Assertion :Sulphuric acid is more visous than water. Reason :Conce...
Text Solution
|
- Assertion : The dipole moment helps to predict whether a molecule is ...
Text Solution
|
- Assertion : Water is a good solvent for ionic compounds but poor one...
Text Solution
|
- Assertion : The atoms in a covalent molecule are said to share el...
Text Solution
|
- Assertion : All F - S - F angle in SF(4) are greater than 90^(@) but ...
Text Solution
|
- Assertion : Both pi(2p(x)) and pi**(2p(x)) MO's have one modal plane...
Text Solution
|