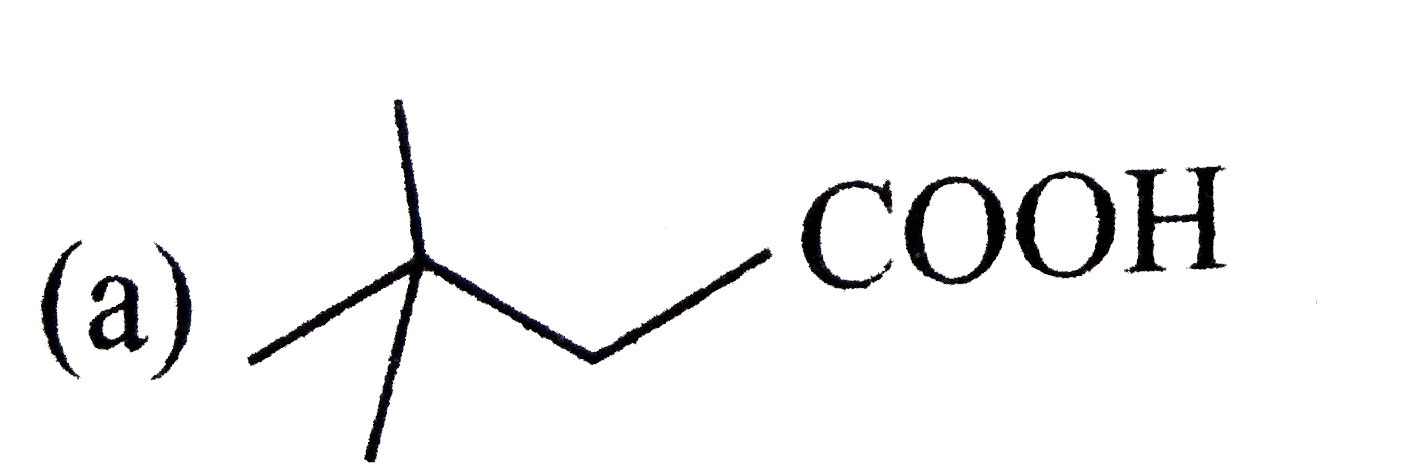
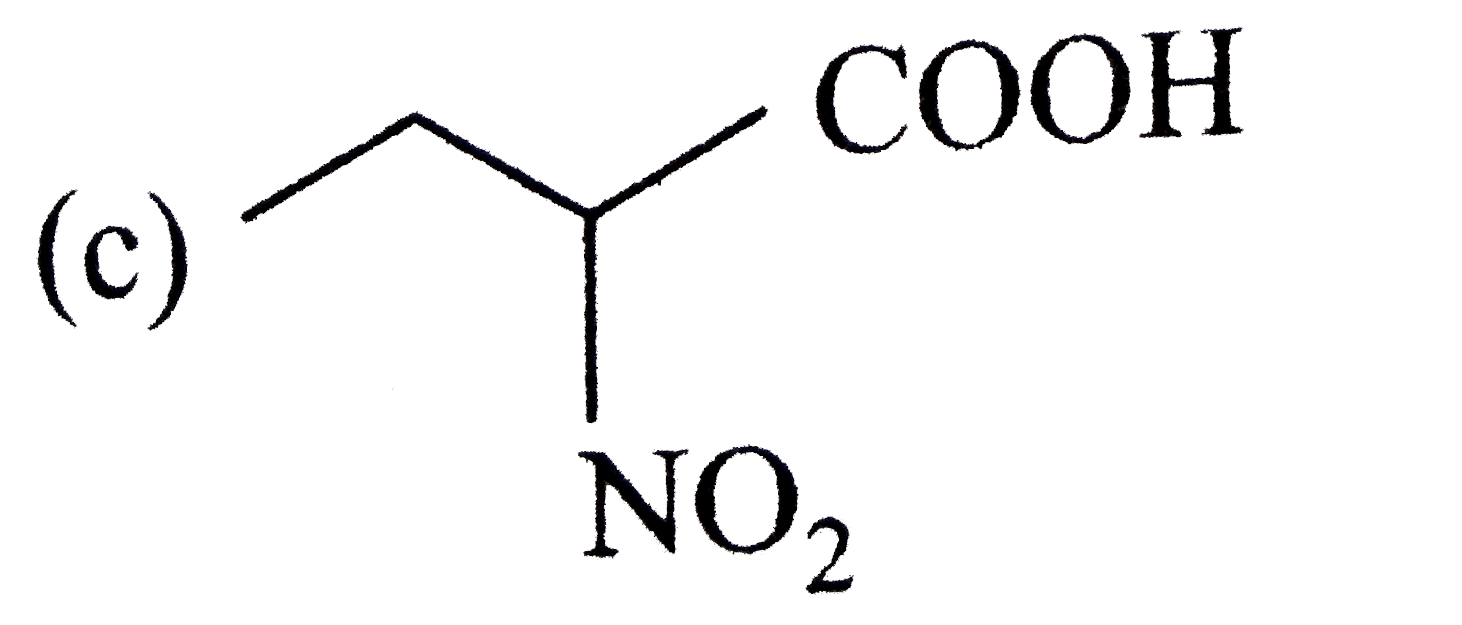
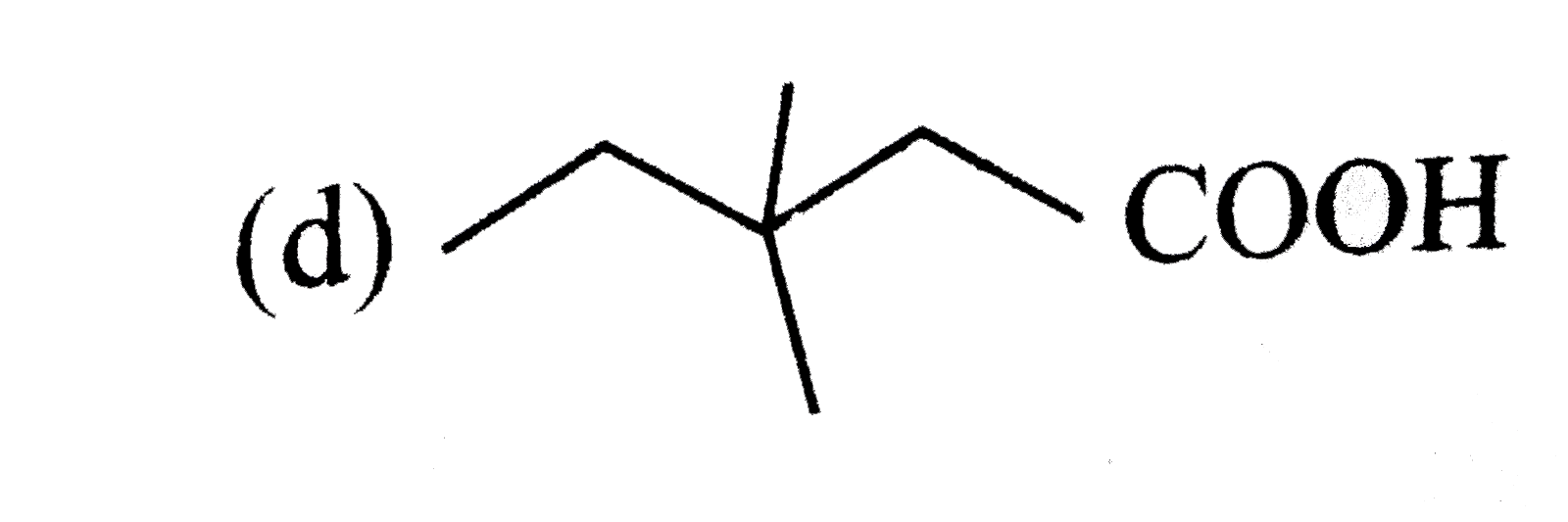
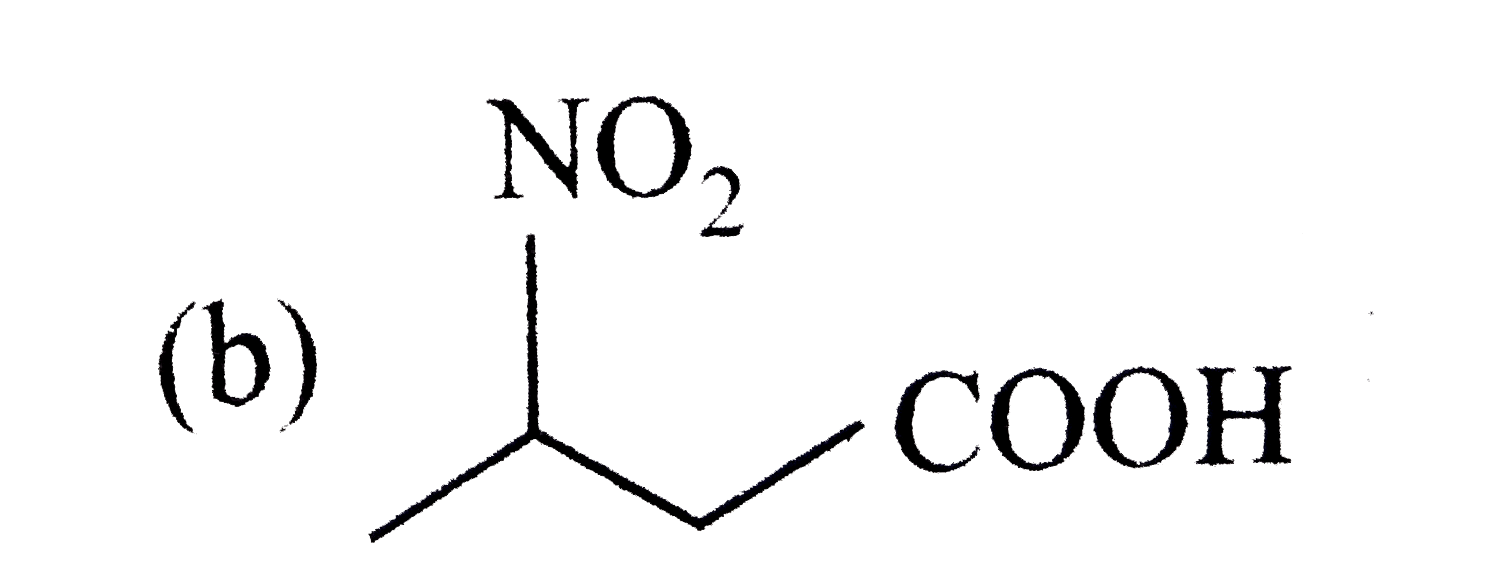A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-HYDROCARBONS-Section D - Chapter End Test
- Which of the following carboxylic acid can undergo deccarboxylation on...
Text Solution
|
- Anti- Markovwnikoff's addtion of HBr is not observed in
Text Solution
|
- Which of the following reactions will yield 2,2-dibromopropane ?
Text Solution
|
- The products formed by the ozonolysis - hydrolysis of compound of form...
Text Solution
|
- When acetylen reacted with hydroxylic acid in presence of HgCl(20 the ...
Text Solution
|
- When propyne reacts with aqueous H(2)SO(4) in the presence of HgSO(4)...
Text Solution
|
- Which one of the following does not dissolve in conc. H(2)SO(4)?
Text Solution
|
- Which one of the following compounds will give in the presence of pero...
Text Solution
|
- Which of the following alkene in acid catalysed hydration form 2-methy...
Text Solution
|
- Which of the following compounds yields only one product on monobromin...
Text Solution
|
- Aqueous solution of the following compounds are electrolysed . Acetyle...
Text Solution
|
- Dehydration of butan -2-ol with conc. H(2)SO(4) gives preferred produc...
Text Solution
|
- CH(3)-C-=C-CH(3)overset(NaNH(2))rarr'X'. What is X ?
Text Solution
|
- Identify the compound 'Y' in the following sequency of reaction HC-=...
Text Solution
|
- Dehydration of 1-butanol gives 2-butene as a major product , by which ...
Text Solution
|
- The prinicipal organic product formed is the reaction : CH(2)=CH(CH(...
Text Solution
|
- Which of the following decolorizes Potassium permanganate?
Text Solution
|
- CH(3)C-=C CH(3) overset((i)X) underset((ii)H(2)O //Zn)rarr X in the...
Text Solution
|
- Which of the following is Friedel - Craft's reaction
Text Solution
|
- Condition for maximum yield of C(2)H(5)Cl is
Text Solution
|
- When ethyl alcohol is heated with red phosphorus and HI, then which o...
Text Solution
|