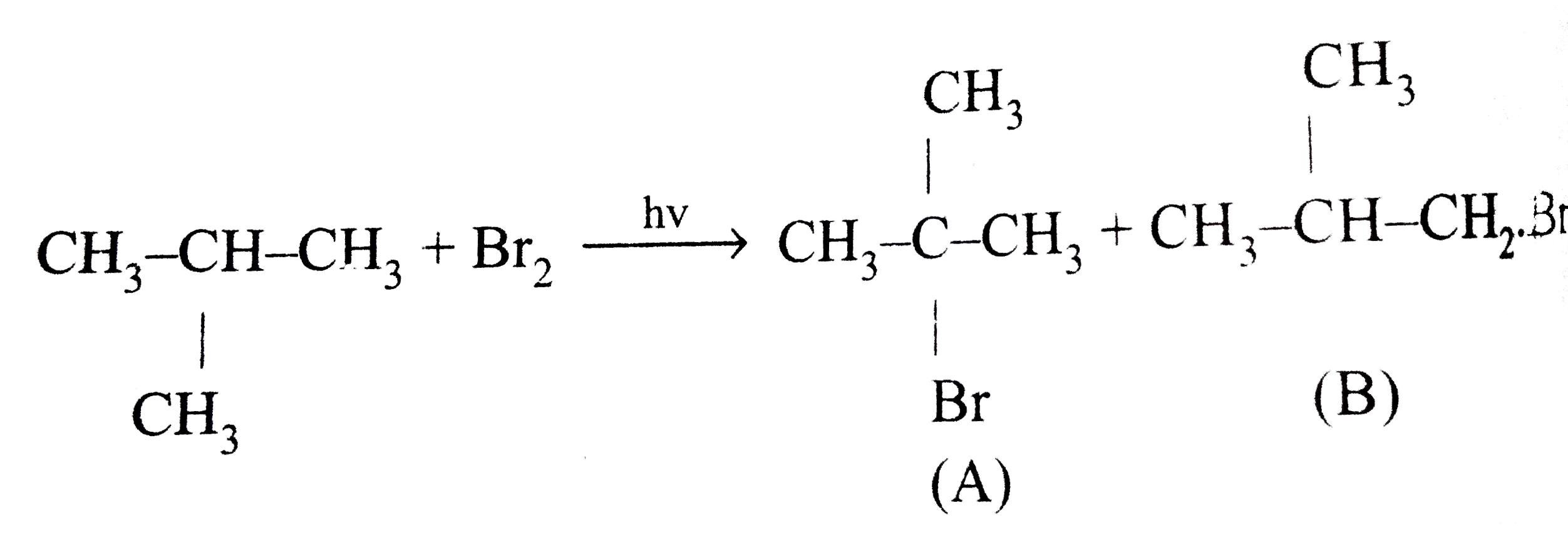
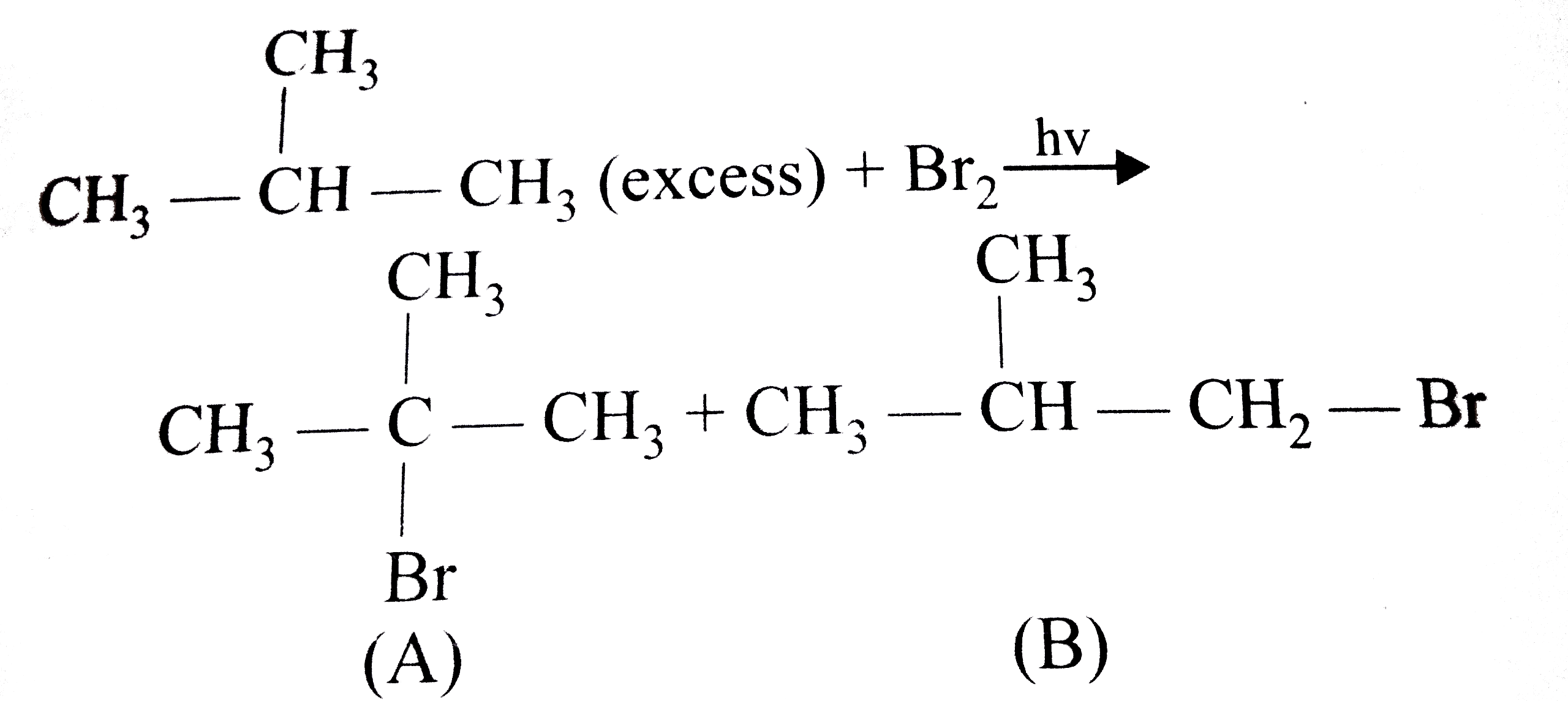A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
A2Z|Exercise Methods Of Preparation Of Alkanes|31 VideosHYDROCARBONS
A2Z|Exercise Method of Preparation of Alkynes|59 VideosHYDROCARBONS
A2Z|Exercise Section D - Chapter End Test|30 VideosENVIRONMENTAL CHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 VideosHYDROGEN
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-HYDROCARBONS-Chemical Properties Of Alkanes
- What is the major monobromination product in the following reaction ?
Text Solution
|
- The hymolytic fission of hydrocarbon results in the formation of
Text Solution
|
- The relative reactivity of 1^(@)H,2^(@)H and 3^(@)H in bromination rea...
Text Solution
|
- Halogenation of alkanes is an example of
Text Solution
|
- What is the major bromination product in the following reaction ? CH...
Text Solution
|
- Chlorination of an alkane involves the attack of
Text Solution
|
- When n- butane is heated iin the presence of AlCl(3)HCl it will be co...
Text Solution
|
- What is true about the reaction given below ? CH(3)-CH=CH-CH(3)+CH(2...
Text Solution
|
- The reactivity of hydrogen atoms attached to carbon atom in the haloge...
Text Solution
|
- Which of the following is oxidised by KMnO(4)?
Text Solution
|
- In which of the following pairs, the bromination of first member is ea...
Text Solution
|
- The addition of tetraethyl lead to petrol
Text Solution
|
- Which of the following cannot be considered as a step of mechanism in ...
Text Solution
|
- Which of the following has maximum boiling point ?
Text Solution
|
- During clhorination of methane to methyl chloride, the propagation ste...
Text Solution
|
- BrCH(2)-CH(2)CH(2)Br reacts with Na in the presence of ether at 100^(@...
Text Solution
|
- Which of the following is not an endothermic reaction ?
Text Solution
|
- Methane reacts with excess of chlorine in diffused sunlight to give th...
Text Solution
|
- A mixture of propene and methane is obtained by the cracking of
Text Solution
|
- A gaeous hydrocation 'X' on reaction with bromine in light forms a mix...
Text Solution
|