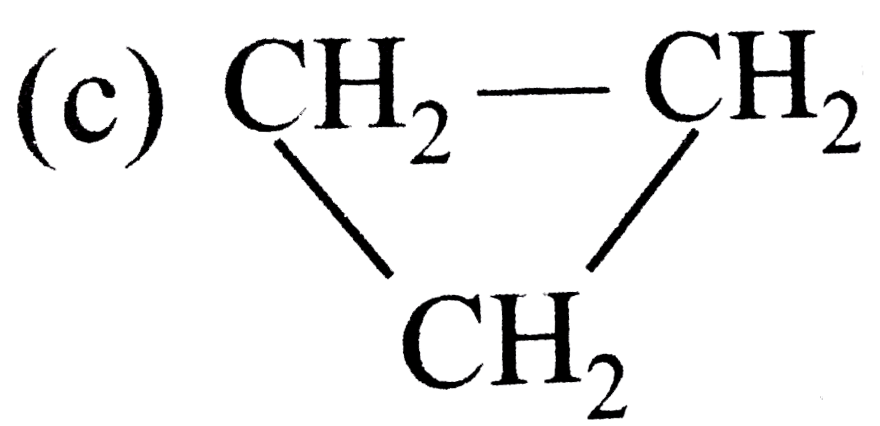A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
A2Z|Exercise Methods Of Preparation Of Alkanes|31 VideosHYDROCARBONS
A2Z|Exercise Method of Preparation of Alkynes|59 VideosHYDROCARBONS
A2Z|Exercise Section D - Chapter End Test|30 VideosENVIRONMENTAL CHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 VideosHYDROGEN
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-HYDROCARBONS-Chemical Properties Of Alkanes
- Which of the following has maximum boiling point ?
Text Solution
|
- During clhorination of methane to methyl chloride, the propagation ste...
Text Solution
|
- BrCH(2)-CH(2)CH(2)Br reacts with Na in the presence of ether at 100^(@...
Text Solution
|
- Which of the following is not an endothermic reaction ?
Text Solution
|
- Methane reacts with excess of chlorine in diffused sunlight to give th...
Text Solution
|
- A mixture of propene and methane is obtained by the cracking of
Text Solution
|
- A gaeous hydrocation 'X' on reaction with bromine in light forms a mix...
Text Solution
|
- The final product of complete oxidation of hydrocarbons is
Text Solution
|
- The appropriate reagent for the transformation
Text Solution
|
- The number of monochloro derivatives of isohexane is ( Only structural...
Text Solution
|
- Which of the following represents the most oxidized form of hydrocarbo...
Text Solution
|
- Iodination of an alkanae is carried out in presence of
Text Solution
|
- What is the chief product obtained when n-butane is treated with Br(2)...
Text Solution
|
- A sample of petrol is a mixture of 30% n- heptane and 70% iso- octane ...
Text Solution
|
- The maximum ease of abstraction of a hydrogen atom by a chlorine atom ...
Text Solution
|
- Propene on reaction with methylen iodide in presence of Zn-Cu couple g...
Text Solution
|
- For the given reaction how many monohalo products are optically active...
Text Solution
|
- Gasoline has composition
Text Solution
|
- Which of the following statement is correct in relation to the halogen...
Text Solution
|
- Of the five isomeric hexanes, the isomer which can give two monochlori...
Text Solution
|