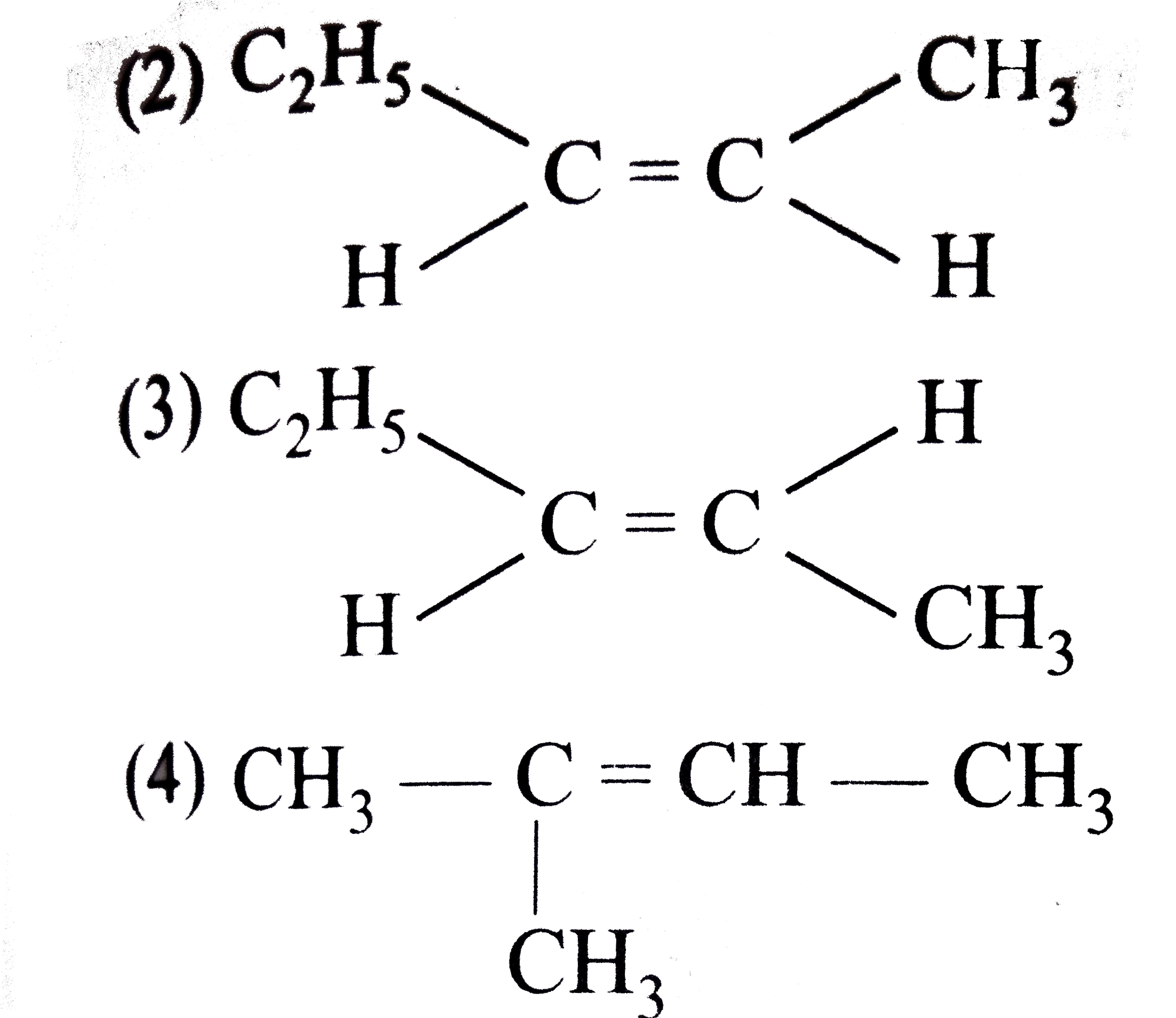
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
A2Z|Exercise Methods Of Preparation Of Alkanes|31 VideosHYDROCARBONS
A2Z|Exercise Method of Preparation of Alkynes|59 VideosHYDROCARBONS
A2Z|Exercise Section D - Chapter End Test|30 VideosENVIRONMENTAL CHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 VideosHYDROGEN
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-HYDROCARBONS-Chemical Properties Of Alkanes
- A mixture of 1- chloropropane and 2- chloropropane which treated with ...
Text Solution
|
- Compound (C) is :
Text Solution
|
- Which is the correct increasing order of the stability of isomers of p...
Text Solution
|
- Which of the following will have zero dipole moment ?
Text Solution
|
- Predict wrong option for stability
Text Solution
|
- But-2- can be obtained by electrolysis of an aqueous solution of
Text Solution
|
- When 1,1,2,2-tetrabomopropane is heated with zinc powder in alcohol, w...
Text Solution
|
- The correct order of alkene reactivity towards an electrophile is ment...
Text Solution
|
- The correct order of reactivity towards electrophilic addition reactio...
Text Solution
|
- Compound 'X' will be :
Text Solution
|
- Which of the following statements is correct ?
Text Solution
|
- P(major) p is :
Text Solution
|
- P(major ),P is :
Text Solution
|
- The correct relative rate of reaction of the given alkenes for any giv...
Text Solution
|
- Which of the following alkene will give (P) on oxymercuration reductio...
Text Solution
|
- P. Identify major product 'P' is :
Text Solution
|
- In which of the following reactions Markownikoff's rule of addition re...
Text Solution
|
- The correct order of rate of following reactions is CH(3)-C=C-CH(3)o...
Text Solution
|
- product X by reaction R.X and R are
Text Solution
|
- Which of the following shows least reactivity towards bormination ?
Text Solution
|