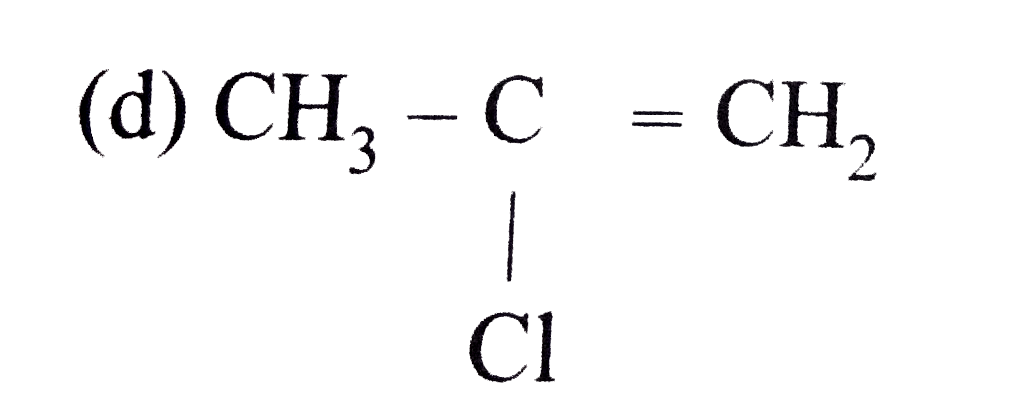A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS
A2Z|Exercise Methods Of Preparation Of Alkanes|31 VideosHYDROCARBONS
A2Z|Exercise Method of Preparation of Alkynes|59 VideosHYDROCARBONS
A2Z|Exercise Section D - Chapter End Test|30 VideosENVIRONMENTAL CHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 VideosHYDROGEN
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-HYDROCARBONS-Chemical Properties Of Alkanes
- CH(3)CH(2)CH(2)C -= CH overset (BH(3),THF)rarroverset(H(2)O //OH^(-))r...
Text Solution
|
- Complete the following reaction
Text Solution
|
- What would be the main product when propene reacts with HBr ?
Text Solution
|
- What would be the main product when propene reacts with HBr is presenc...
Text Solution
|
- Ethene reacts with HOCl to form
Text Solution
|
- What would be the product when ethen is oxidised with cold dil. KMnO(4...
Text Solution
|
- Propene reacts with Cl(20 at 500^(@)C the products is formed
Text Solution
|
- Anti- Markovwnikoff's addtion of HBr is not observed in
Text Solution
|
- At low temperatures, the slow addition of molecular bromine to CH(2)=C...
Text Solution
|
- Reactivity of alkenes towards HX decreases in the order
Text Solution
|
- CH(3)CH=CH(2)overset(DB(3))underset(H(2)O // OH^())rarr productX X...
Text Solution
|
- A will have configuration
Text Solution
|
- Complete the following reaction
Text Solution
|
- A , which is true about this reaction ?
Text Solution
|
- A,B and C are
Text Solution
|
- Complete the following reaction
Text Solution
|
- Dehydration of 2,2,3,4,4-pentamethy 1-3-pentanol gave two alkenes A an...
Text Solution
|
- Identify 'Z' in the following reaction series, CH(3)CH(2)CH(2)Brover...
Text Solution
|
- Propene reacts with Cl(2 at 500^(@)C the products is formed
Text Solution
|
- When HCl gas is passed through propene in the presence of benzoyl pero...
Text Solution
|