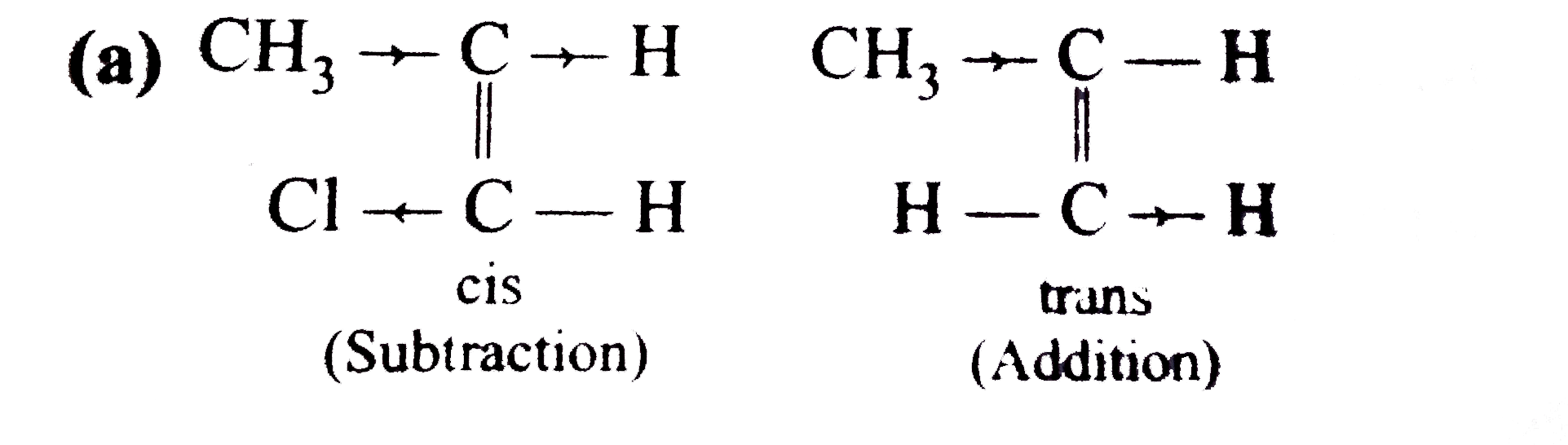A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-HYDROCARBONS-Section B - Assertion Reasoning
- Assertion : When double and triple bonds are in conjugation, addition ...
Text Solution
|
- Assertion-Addition of bromine to trans-2-butene yields meso-2,3-dibrom...
Text Solution
|
- Assertion : Cyclohexane floats over water. Reason: Cyclohexane alway...
Text Solution
|
- Assertion :Alkeanes having more than three carbon atoms exhibit chain ...
Text Solution
|
- Assertion : Cis-1,3 dihydroxy cyclohexane exists in chair conformation...
Text Solution
|
- Assertion : 2,3- dimethyl but-2-ene decolorizes Br(2) water. Reason:...
Text Solution
|
- Assertion : Reaction of HCl with But-2- ene in the presence or absence...
Text Solution
|
- Assertion : Reaction of tert- butychloride with Na gives 2,2,3,3- tetr...
Text Solution
|
- Assertion : 2,3-dimethyl but-2-ene is more stable than but-2-ene. Re...
Text Solution
|
- Assertion: Trans-2-chloro propene has higher dipole moment than cis-2-...
Text Solution
|
- Assertion : Dimethyl sulphide is commonly used for the reduction of a...
Text Solution
|
- Assertion : Iodination of alkane is carried out in presence of iodic a...
Text Solution
|
- Assertion: C(6)H(6) does not decolourize Br(2) water. Reason: All t...
Text Solution
|
- Assertion : Tertiary butyl benzene on oxidation give benzoic acid. R...
Text Solution
|
- Assertion: Benzene is reactive while inorganic benzene is unreactive ...
Text Solution
|
- Assertion : Alkyl benzene is not prepared by Friedel- Crafts alkylatio...
Text Solution
|
- Assertion : Tropylium cation is aromatic in nature Reason : The only...
Text Solution
|
- Assertion : CH(3)-C-=C-CH(3) is more reactive for electrophilic additi...
Text Solution
|
- Assertion : Addition of HBr in the presence of peroxide on 1-methyl c...
Text Solution
|
- Assertion : Stability of alkene is governed by hyper conjugation Rea...
Text Solution
|