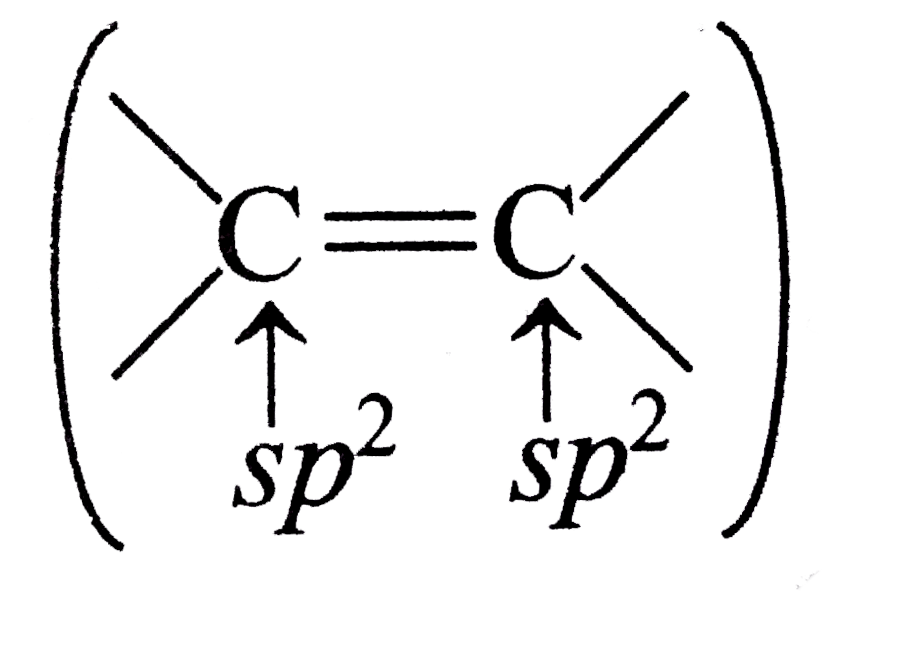
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
A2Z-HYDROCARBONS-Assertion-Reasoning Questions
- Assertion : Benzene removes a butter stain from a table cloth. Reaso...
Text Solution
|
- Assertion : Ethane is much less reactive than ethene. Reason : Bond ...
Text Solution
|
- Assertion : A mixture of HNO(3) and H(2)SO(4) is used for the nitratio...
Text Solution
|
- Assertion (A) : All the C atoms of but-2-ene lie in one plane Reason...
Text Solution
|
- Assertion : trans - Pent -2- ene is polar but trans - but -2- ene is n...
Text Solution
|