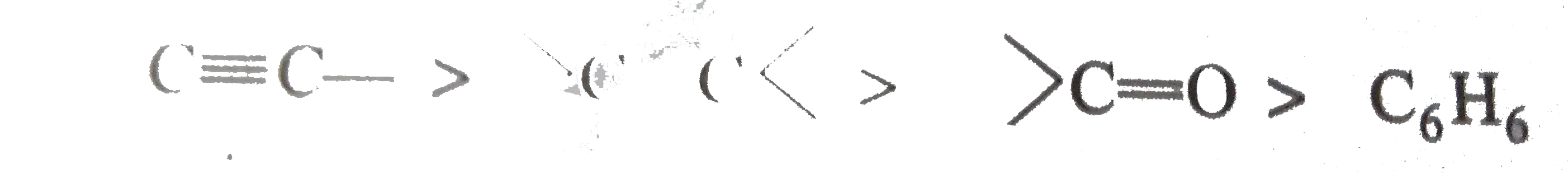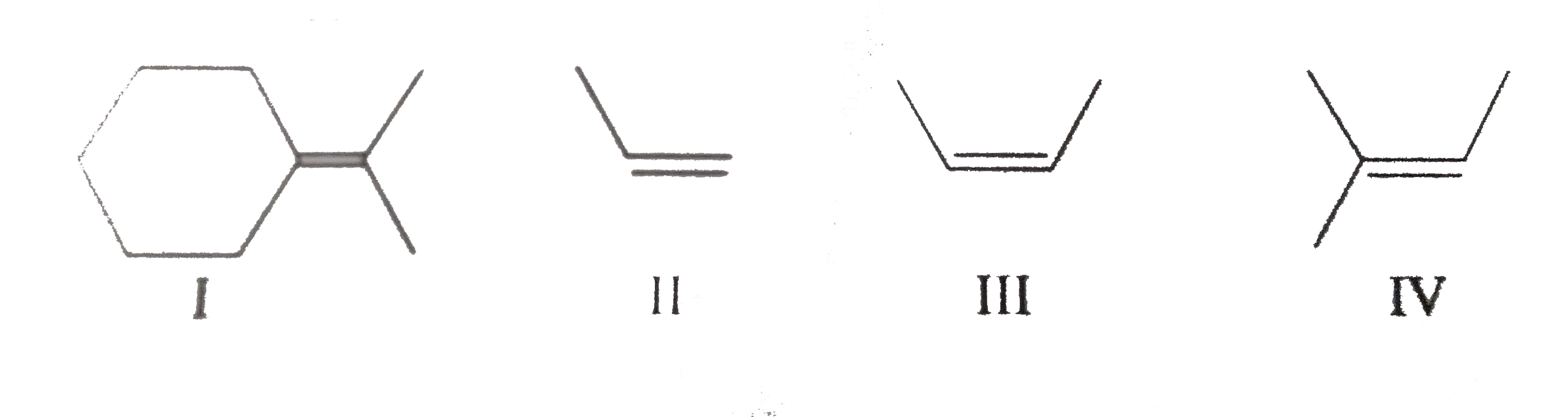Similar Questions
Explore conceptually related problems
Recommended Questions
- Hydrogenation of alkenes and alkynes takes place in presence of certai...
Text Solution
|
- Alkyne can be reduced to alkenes by hydrogenation in presence of
Text Solution
|
- Hydrogenation of alkenes and alkynes takes place in presence of certai...
Text Solution
|
- Hydrogenation of alkenes and alkynes takes place in presence of certai...
Text Solution
|
- Hydrogenation of alkenes and alkynes takes place in presence of certai...
Text Solution
|
- Hydrogenation of alkenes and alkynes takes place in presence of certai...
Text Solution
|
- Hydrogenation of alkenes and alkynes takes place in presence of certai...
Text Solution
|
- Hydrogenation of alkenes and alkynes takes place in presence of certai...
Text Solution
|
- Hydrogenation of alkenes and alkynes takes place in presence of certai...
Text Solution
|