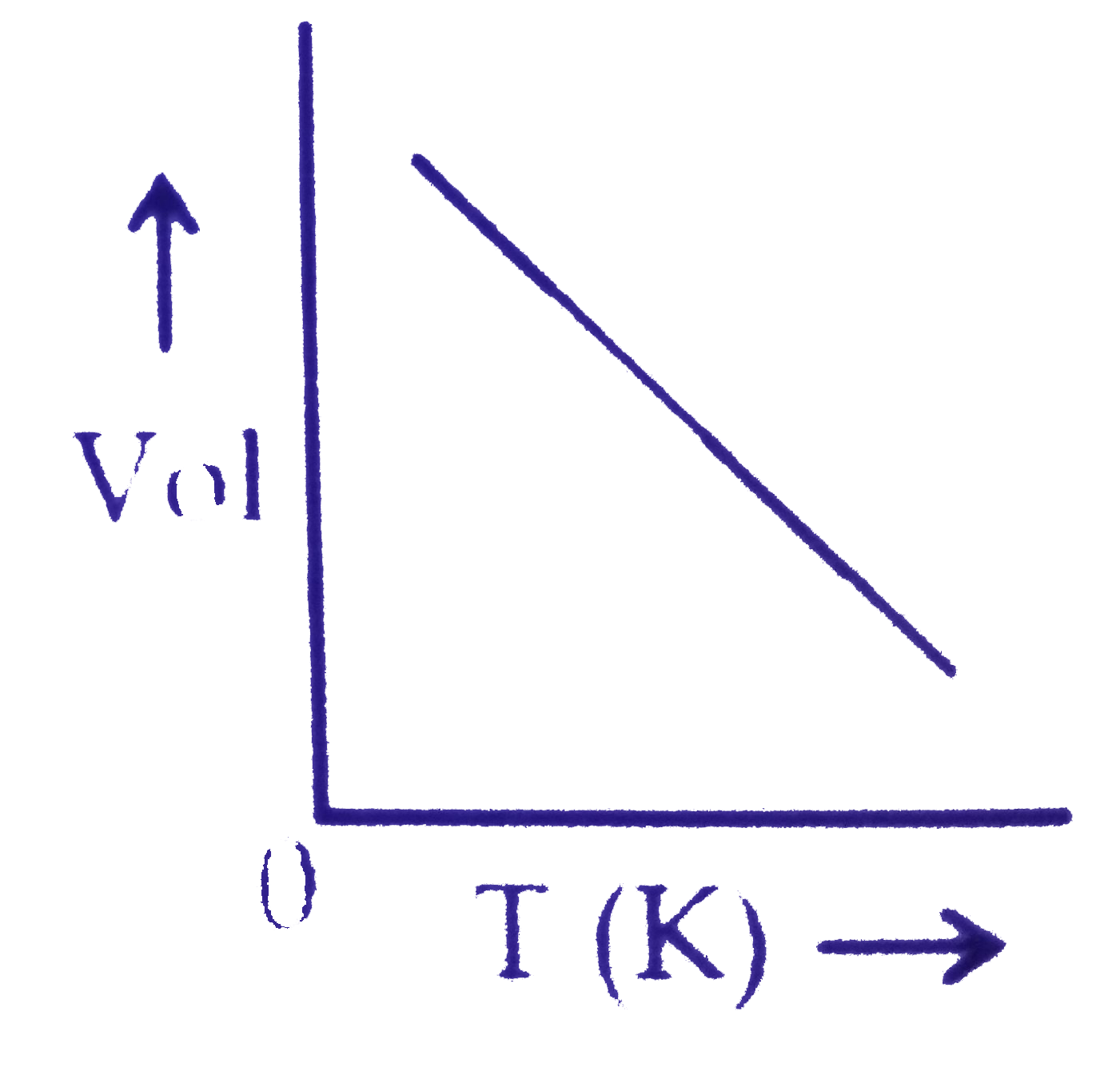
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDS
MARVEL PUBLICATION|Exercise Ideal Behaviour and Gas Equation and Deviation from Ideal Behaviour|40 VideosSTATES OF MATTER : GASES AND LIQUIDS
MARVEL PUBLICATION|Exercise Liquefaction of Gases Critical Teperature Pressure and Volume|22 VideosSTATES OF MATTER : GASES AND LIQUIDS
MARVEL PUBLICATION|Exercise Gaseous State|6 VideosSOLID STATE
MARVEL PUBLICATION|Exercise Multiple choice questions|183 VideosSURFACE CHEMISTRY
MARVEL PUBLICATION|Exercise TEST YOUR GRASP|35 Videos
Similar Questions
Explore conceptually related problems
MARVEL PUBLICATION-STATES OF MATTER : GASES AND LIQUIDS -Boyle s Law Charles Law Gay-Lussacs Law and Avogadros Law
- The mountaineers carry oxygen gas cylinders with them while climbing h...
Text Solution
|
- If V(0) is the volume of a given mass of gas at 273K at a constant pre...
Text Solution
|
- The correct representation of Charle's law is given in
Text Solution
|
- At of the following does not showexpliently the relationship between B...
Text Solution
|
- If the absolute temperature of a gas is doubled and the pressure is re...
Text Solution
|
- The molar volume of CO(2) is maximum at
Text Solution
|
- There is 10 litre of a gas at STP Which of the following new condition...
Text Solution
|
- Five grams each of the following gases at 87^(@)C and 750 mm pressure ...
Text Solution
|
- According to Charles' law, at constant pressure, 100 ml of a given mas...
Text Solution
|
- A sealed tube which can withstand a pressure of 3 atmosphere is fille...
Text Solution
|
- 400 cm^(3) of oxygen at 27^(@)C were cooled to -3^(@)C without change ...
Text Solution
|
- An electron tube was sealed off during manufacture at a pressure of 1....
Text Solution
|
- 16 g of oxygen and 3g of hydrogen are mixed and kept at 760 mm of Hg p...
Text Solution
|
- 2.8 g of N(2) 0.40 g of H(2) and 6.4 g of O(2) are placed in a contain...
Text Solution
|
- One litre of a gas weights 2 g at 300 K and 1 atm pressure. If the pre...
Text Solution
|
- The closed containers of the same capacity and at the same temperature...
Text Solution
|
- V vs T curves at constant pressure P(1) and P(2) for an ideal gas are ...
Text Solution
|
- Containers A,B and C of equal volume contain oxygen neon and methane r...
Text Solution
|
- Pressure remaining the same, the volume of a given mass of an ideal ga...
Text Solution
|
- At constant temperature, in a given mass of an ideal gas -
Text Solution
|