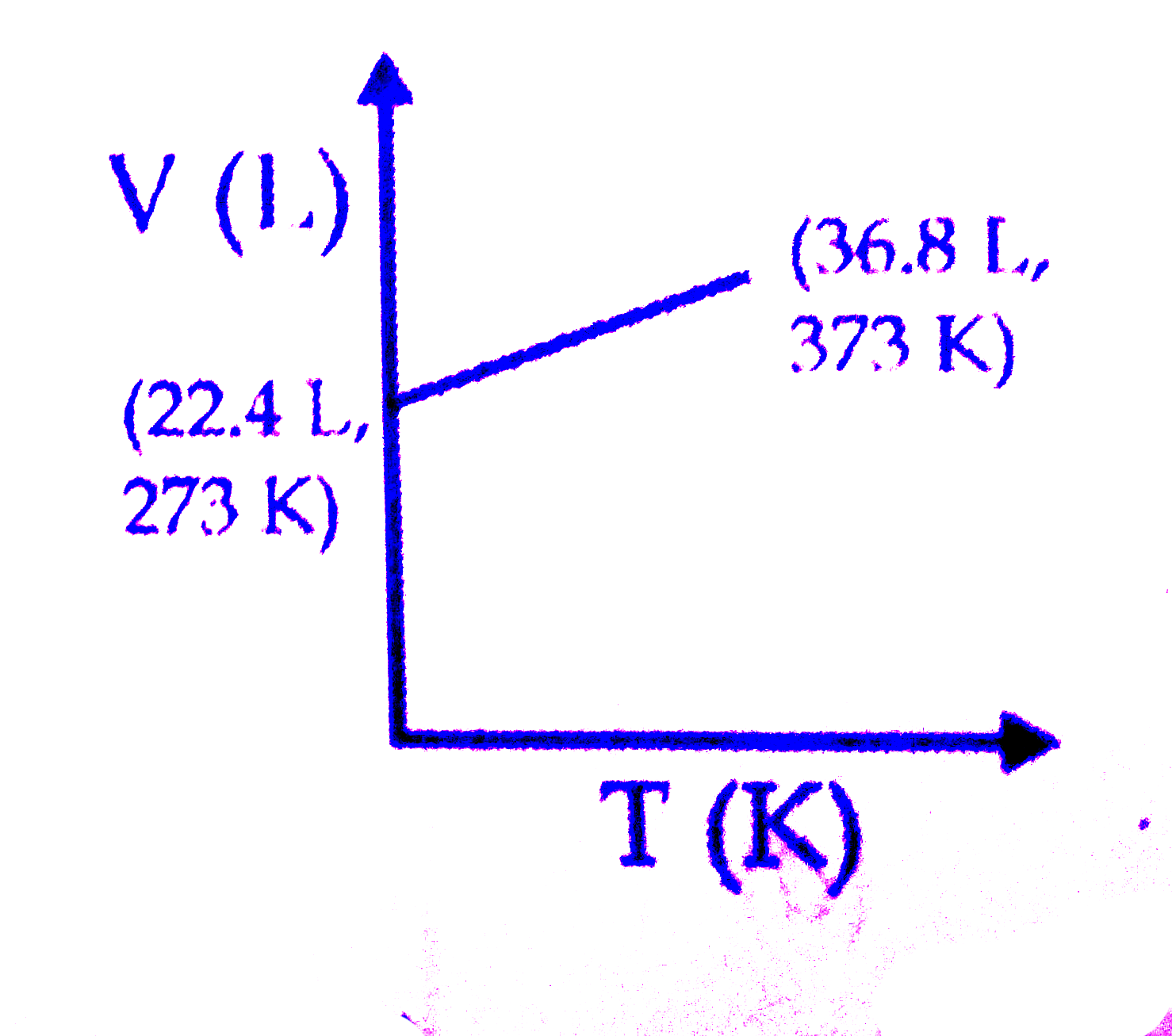
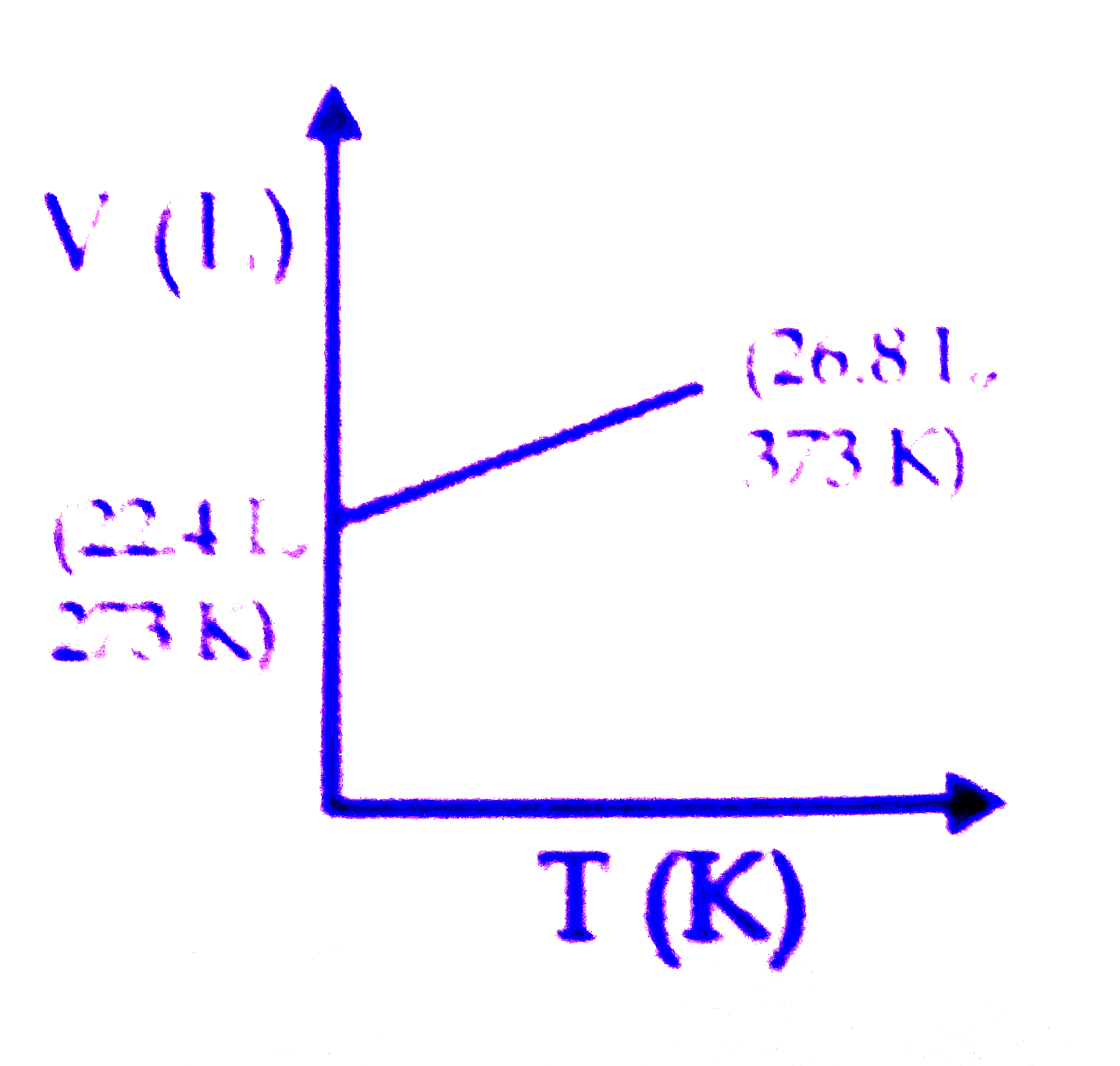
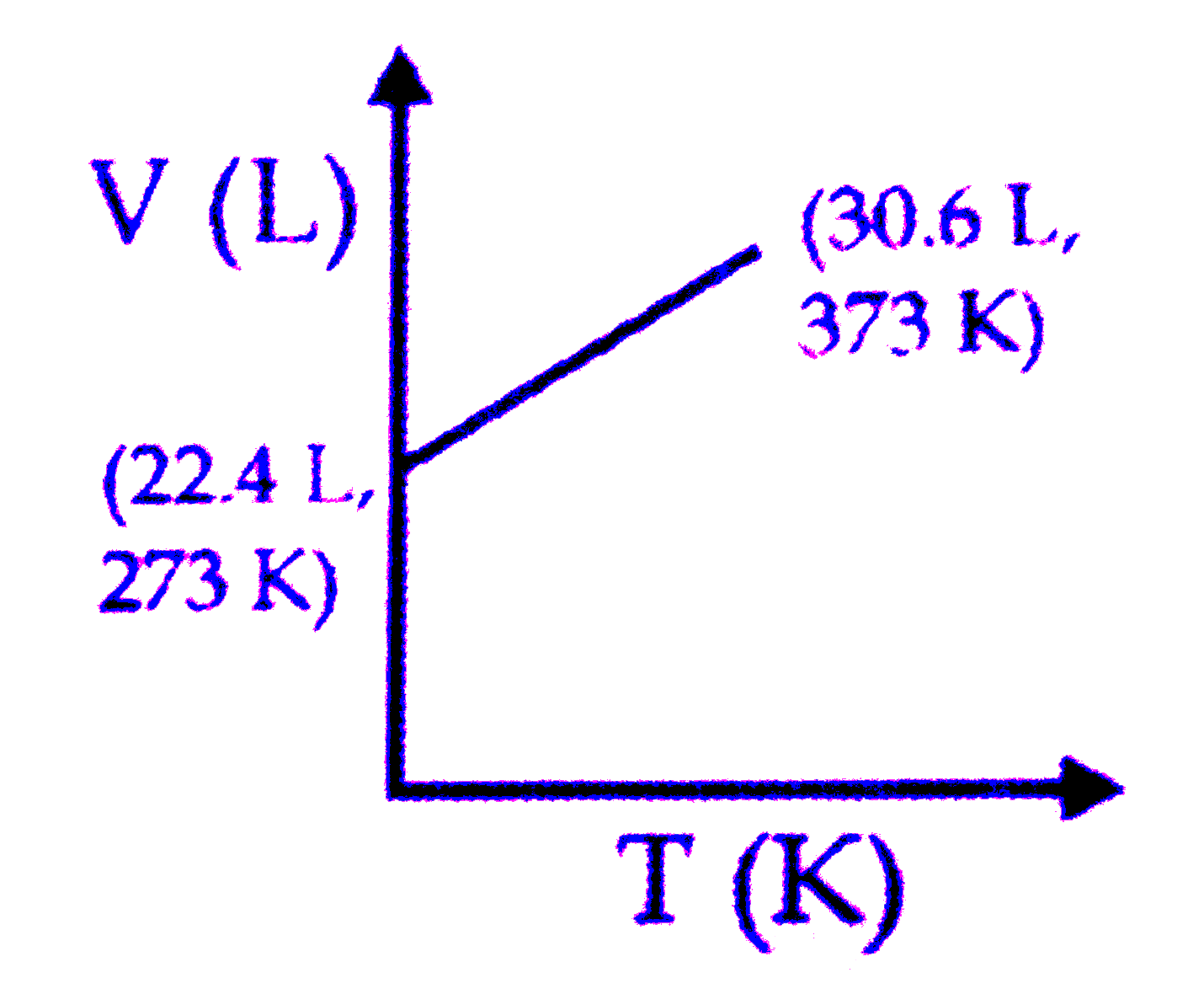
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STATES OF MATTER : GASES AND LIQUIDS
MARVEL PUBLICATION|Exercise Ideal Behaviour and Gas Equation and Deviation from Ideal Behaviour|40 VideosSTATES OF MATTER : GASES AND LIQUIDS
MARVEL PUBLICATION|Exercise Liquefaction of Gases Critical Teperature Pressure and Volume|22 VideosSTATES OF MATTER : GASES AND LIQUIDS
MARVEL PUBLICATION|Exercise Gaseous State|6 VideosSOLID STATE
MARVEL PUBLICATION|Exercise Multiple choice questions|183 VideosSURFACE CHEMISTRY
MARVEL PUBLICATION|Exercise TEST YOUR GRASP|35 Videos
Similar Questions
Explore conceptually related problems
MARVEL PUBLICATION-STATES OF MATTER : GASES AND LIQUIDS -Boyle s Law Charles Law Gay-Lussacs Law and Avogadros Law
- 600 cc of a gas at a pressure of 750 mm is compressed to 500 cc. Takin...
Text Solution
|
- At 25^(@)C and 730 mm pressure, 380 mL of dry oxygen was collected. If...
Text Solution
|
- Which of the following volume-temperature (V-I) plots represents the b...
Text Solution
|
- At 27^(@)C, a ges is compressed to half of its volume . To what temper...
Text Solution
|
- The temperature of a certain mass of a gas was increased from 29^(@) C...
Text Solution
|
- If P,V, and T represent pressure, volume and temperature of the gas, t...
Text Solution
|
- Air at sea level is dense. This is a practical application of
Text Solution
|
- If 20 cm^(3) gas at 1 atm is expanded to 50 cm^(3) at constant T, then...
Text Solution
|
- Which of the following graph represents Boyle's law ?
Text Solution
|
- At constant pressure , the volume of fixed mass of an ideal gas is dir...
Text Solution
|
- Which of the following expression at constant pressure represents Char...
Text Solution
|
- Use of hot air ballons in sports and meteorological observations in a...
Text Solution
|
- A certain sample of gas has a volume of 0.2 litre measured at 1 atm pr...
Text Solution
|
- One gram mole of a gas at NTP occupies 22.4 L. This fact is derived fr...
Text Solution
|
- The density of a gas at 27^(@)C and 1 atm is d. Pressure remaining con...
Text Solution
|
- Equal volumes of gases at the same temperature and pressure contain eq...
Text Solution
|
- 4.4 g of a gas at STP occupies a volume of 2.24 L. The gas can be :
Text Solution
|
- The density of O(2) is 16 at STP. At what temperature (in .^@C) its de...
Text Solution
|
- 500mL of NH(3) contains 6.02xx10^(23) molecules at STP. How many mole...
Text Solution
|
- If molecular mass of O(2) and SO(2) are 32 and 64 respectively. If one...
Text Solution
|