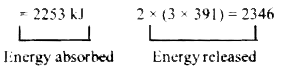A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS AND ENERGETICS
MARVEL PUBLICATION|Exercise MULTIPLE CHOICE QUESTIONS (Thermochemical Equations)|16 VideosCHEMICAL THERMODYNAMICS AND ENERGETICS
MARVEL PUBLICATION|Exercise MULTIPLE CHOICE QUESTIONS (Types of Heat of Reactions)|22 VideosCHEMICAL THERMODYNAMICS AND ENERGETICS
MARVEL PUBLICATION|Exercise MULTIPLE CHOICE QUESTIONS (Heat of Reaction)|18 VideosCHEMICAL KINETICS
MARVEL PUBLICATION|Exercise TEST YOUR GRASP|50 VideosCHEMISTRIY IN EVERYDAY LIFE
MARVEL PUBLICATION|Exercise TEST YOUR GRAPS|24 Videos
Similar Questions
Explore conceptually related problems
MARVEL PUBLICATION-CHEMICAL THERMODYNAMICS AND ENERGETICS-MULTIPLE CHOICE QUESTIONS (Bond Enthalpy)
- The bond energy of H(2) is 436.4 kJ. This means that
Text Solution
|
- In the dissociation of CH(4(g))
Text Solution
|
- Given the bond energies N-N , N-H and H-H bond are 945, 436 and 391KJm...
Text Solution
|
- Heat evolved in the reaction H(2)+Cl(2) rarr 2HCl is 182 kJ Bond ene...
Text Solution
|
- The bond energies of F(2),Cl(2),Br(2) and I(2) are 155.4, 243.6, 193.2...
Text Solution
|
- The bond energy of an O -H bond is 109 K. cal "mole"^(-1). When a mole...
Text Solution
|
- The bond energy of C-H bond in CH(4) from the following data: C((s))...
Text Solution
|
- If the heat of formation of MH(4) is -1600 kJ, the bond energy of M-H ...
Text Solution
|
- The bond energy of O-H bond is ''Y'' J "mol"^(-1). When one mole of wa...
Text Solution
|
- The bond dissociation energy of gaseous H(2), Cl(2) and HCl are 104, 5...
Text Solution
|