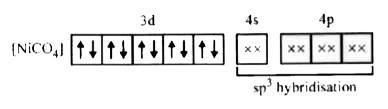A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MARVEL PUBLICATION-CO-ORDINATION COMPOUNDS-TEST YOUR GRASP
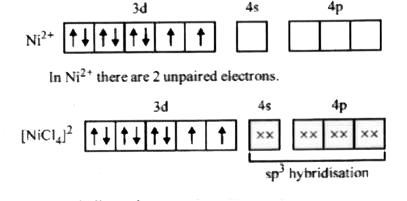
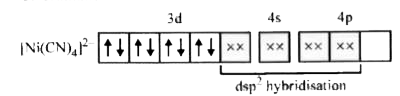
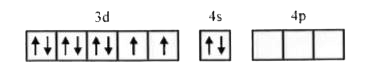
- Amongst Ni(CO)(4),[Ni(CN)(4)]^(2-) and NiCl(4)^(2-):
Text Solution
|
- Which of the following is odd one out ?
Text Solution
|
- An example for a double salt is
Text Solution
|
- CuSO(4) dissolves in NH(3) due to formation of
Text Solution
|
- K(4)[Fe(CN)(6)] is a
Text Solution
|
- Some salts although containing two different metallic elements give te...
Text Solution
|
- The EAN of iron in [Fe(CN)(6)]^(3-) is
Text Solution
|
- Coordination number of Ni in [Ni(C2O4)3]^(4-) is:
Text Solution
|
- According to Lewis the ligands are
Text Solution
|
- According to the postulates of Werner for cooedination compounds
Text Solution
|
- In[Co(NH(3))(6)]Cl(3),the number of covalent bonds is
Text Solution
|
- Chemical formula for in iron(III) hexacyanoferrate(II) is
Text Solution
|
- IUPAC name of Na(3)[Co(NO(2))(6)] is
Text Solution
|
- Ligands in a complex salt are:
Text Solution
|
- The IUPAC name of K(3)[Ir(C(2)O(4))(3)] is
Text Solution
|
- In [Cr(C(2)O(4))(3)]^(3-), the isorerism shown is
Text Solution
|
- Which of the following complex will show geometrical as well as optica...
Text Solution
|
- A coordination compound of cobalt has the molecular, formula containin...
Text Solution
|
- The number of isomers exhibited by [Cr(NH(3))(3)Cl(3)] is
Text Solution
|
- Which of the following will give maximum number of isomer ?
Text Solution
|
- The compounds [PtCl(2)(CH(3))(4)]Br(2) and [PtBr(2)(NH(3))(4)]Cl(2) co...
Text Solution
|