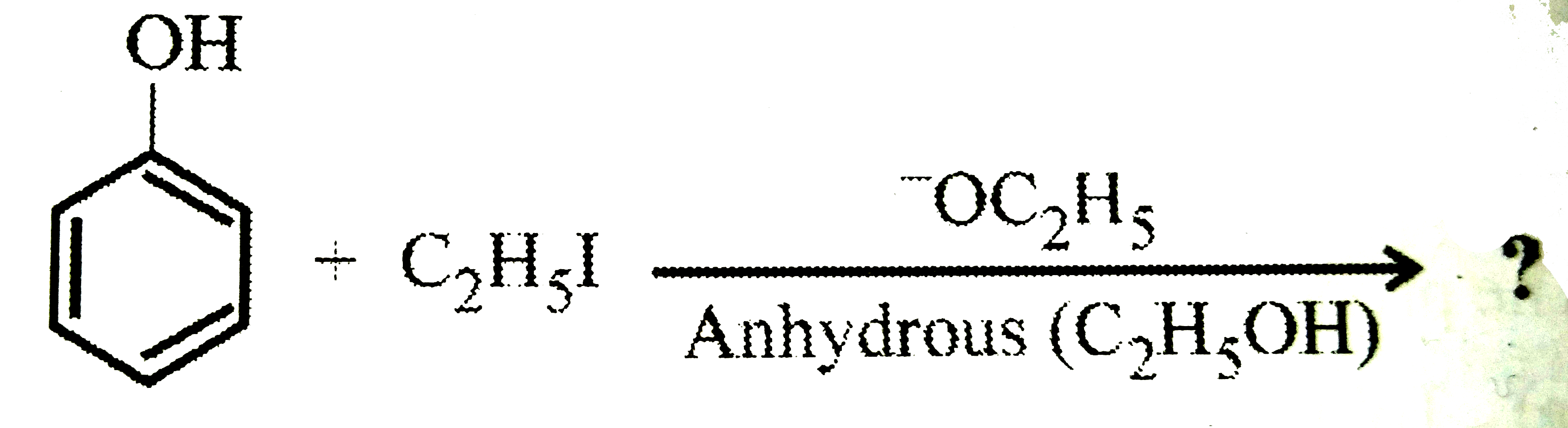
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
MARVEL PUBLICATION-ALCOHOL,PHENOLS AND ETHERS -Test your Grasp : Ethers
Text Solution
|
- Ethers contain which of the following ?
Text Solution
|
- Cold HI reacts with CH3-O-C2H5 to give
Text Solution
|
- Ethers are considered as
Text Solution
|
- Which one of the following pairs of compounds represent the metamers ...
Text Solution
|
- Which one of the following compounds is not isomeric with Ethoxyethan...
Text Solution
|
- The IUPAC name of C2H5-O-CH2-CH(CH3)2 is
Text Solution
|
- Methoxy methane in the IUPAC name of
Text Solution
|
- Which of the following is not true about diethyl ether ?
Text Solution
|
- Diazomethane reacts with ethyl alcohol to give
Text Solution
|
- The reaction CH3I+CH3ONa to CH3-O-CH3+NaI is
Text Solution
|
- An organic compound X reacts with exces of HI to give Y. Further Y can...
Text Solution
|
- Intermolecular dehydration of alcohols gives
Text Solution
|
- Which one of the following alkyl halide gives best yield in Williams...
Text Solution
|
- Which one of the following ethers cannot be prepared by using diazomet...
Text Solution
|
- Bromo ethane reacts with to give ethoxyethane .
Text Solution
|
- The continuous etherification process will generally give
Text Solution
|
- Ethers may behaves as
Text Solution
|
- Solvent ether in chemical reaction is
Text Solution
|
- A simple ether is
Text Solution
|
- Ethers are used as
Text Solution
|