A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MARVEL PUBLICATION-ALDEHYDES,KETONES AND CARBOXYLIC ACIDS-CARBOXYLIC ACIDS
- Which factor/s will increase the reactivity of group? (i)Presence ...
Text Solution
|
- In the case of carboxylic acids,the alpha-carbon is the carbon:
Text Solution
|
- General formula for mono-carboxylic acid is :
Text Solution
|
- Aliphatic carboxylic acid are functional isomers of
Text Solution
|
- Compound having molecular formula C(3)H(6)O(2) is:-
Text Solution
|
- Which of the following formula represent chloroethanoic acid ?
Text Solution
|
- Fatty acids are
Text Solution
|
- General formula for mono-carboxylic acid is :
Text Solution
|
- Which of the following is example of tricarboxylic acid ?
Text Solution
|
- Glacial acetic acid is the same as :
Text Solution
|
- Which of the follwong compound does not have a carboxyl group.
Text Solution
|
- Acetic acid is a weak acid because :
Text Solution
|
- Which one of the following is ethanoic acid ?
Text Solution
|
- The common name of CH(3)(CH(2))(16)COOH acid is :
Text Solution
|
- The I.U.P.A.C. name of CH(3)-CH(2)-underset(CH(3))underset(|)(C)H-CH(2...
Text Solution
|
- The I.U.P.A.C. name of lactic acid CH(3)CHOHCOOH is :
Text Solution
|
- The IUPAC name of the compound :
Text Solution
|
- Which of the following acids occur in ants ?
Text Solution
|
- Most of the carboxylic acids exists as dimer in
Text Solution
|
- Acetic acid can be separated from aqeous solution of acetic acid,at wh...
Text Solution
|
- Which of the following is isobutyric acid?
Text Solution
|
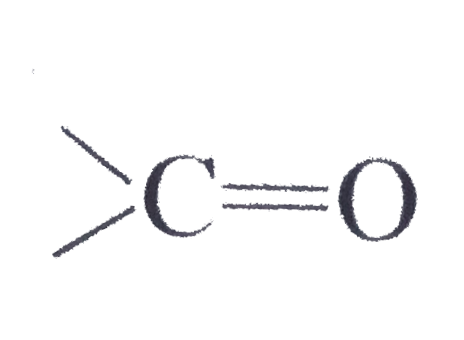 group?
group?