A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MARVEL PUBLICATION-ATOMS, MOLECULES AND NUCLEI -TEST YOUR GRASP
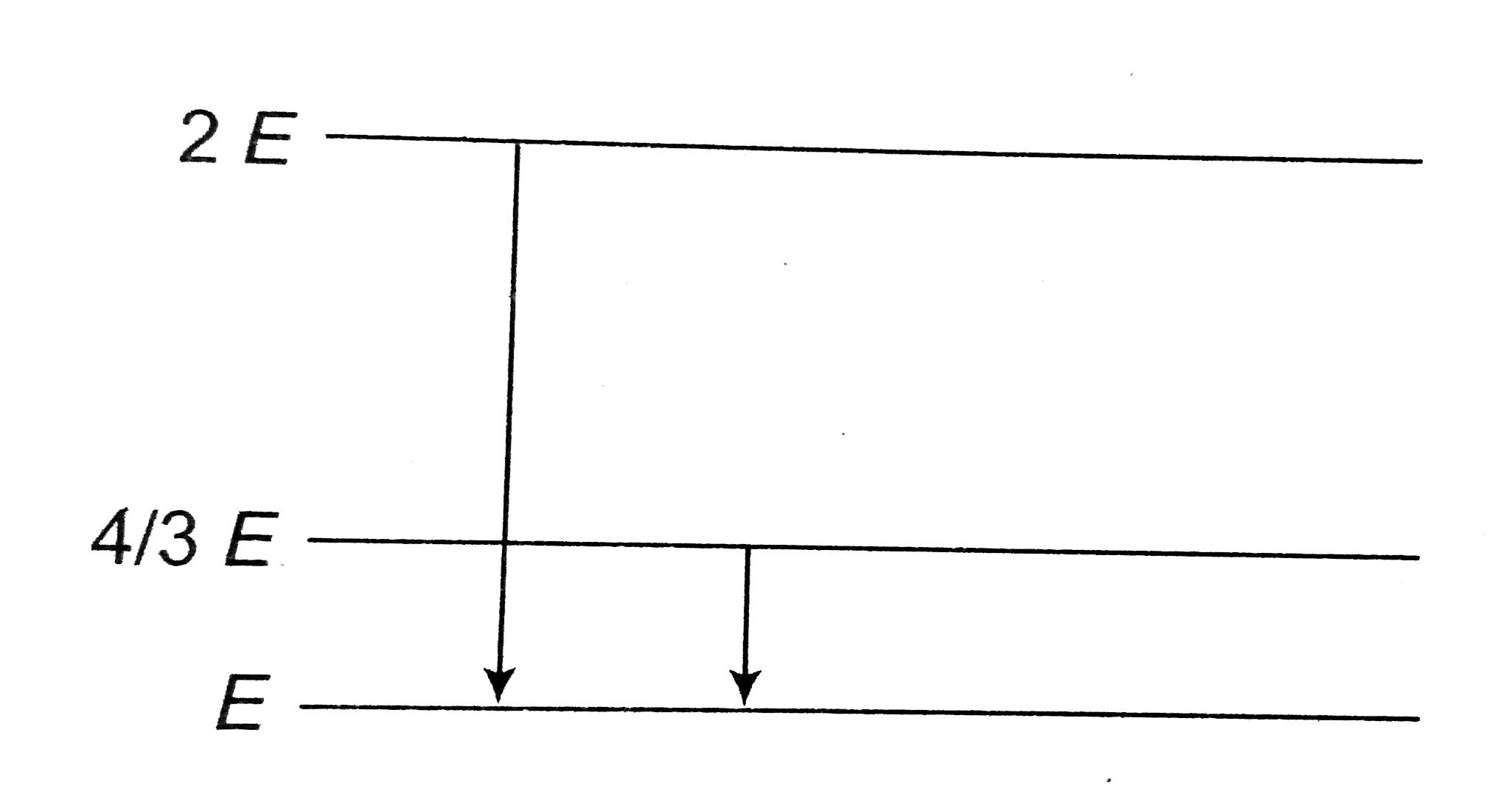
- The follwing diagram indicates the energy levels of a certain atom whe...
Text Solution
|
- According to Rutherford's atom model, the electrons revolving round th...
Text Solution
|
- The velocity of an electron in the first Bohr orbit of hydrogen atom i...
Text Solution
|
- The radius of the orbital of electron in the hydrogen atom 0.5 Å. The ...
Text Solution
|
- The radius of hydrogen atom in its ground state is 5.3 xx 10^-11 m. Af...
Text Solution
|
- In hydrogen atom, if the difference in the energy of the electron in n...
Text Solution
|
- The series limit of Balmer series is 6400 Å. The series limit of Pasch...
Text Solution
|
- An electron jumps from the 3rd orbit to the ground orbit in the hydrog...
Text Solution
|
- If the following atoms and molecylates for the transition from n = 2 t...
Text Solution
|
- The diagram shows the energy levels for an electron in a certain atom....
Text Solution
|
- The de-Broglie wavelength of a particle having a momentum of 2xx10^(-2...
Text Solution
|
- What will be the ratio of de - Broglie wavelengths of proton and alpha...
Text Solution
|
- de-Broglie wavelength associated with an electron accelerated through ...
Text Solution
|
- An electtron and a photon have same wavelength . If p is the moment of...
Text Solution
|
- An X ray tube is operated at an accelerating potential of 40 kV. What ...
Text Solution
|
- A The wavelength of the Kalpha line ofthe characteristic X rays emitt...
Text Solution
|
- In the following reaction. .12 Mg^24 + .2He^4 rarr .14 S i^X +.0 n^1...
Text Solution
|
- The radius of germanium (Ge) nuclide is measured to be twice the radiu...
Text Solution
|
- The binding energy per nucleon is maximum in the case of.
Text Solution
|
- The binding energy per nucleon of O^16 is 7.97 MeV and that of O^17 is...
Text Solution
|
- In any fission the ratio ("mass of fission produts")/("mass of paren...
Text Solution
|