Similar Questions
Explore conceptually related problems
Recommended Questions
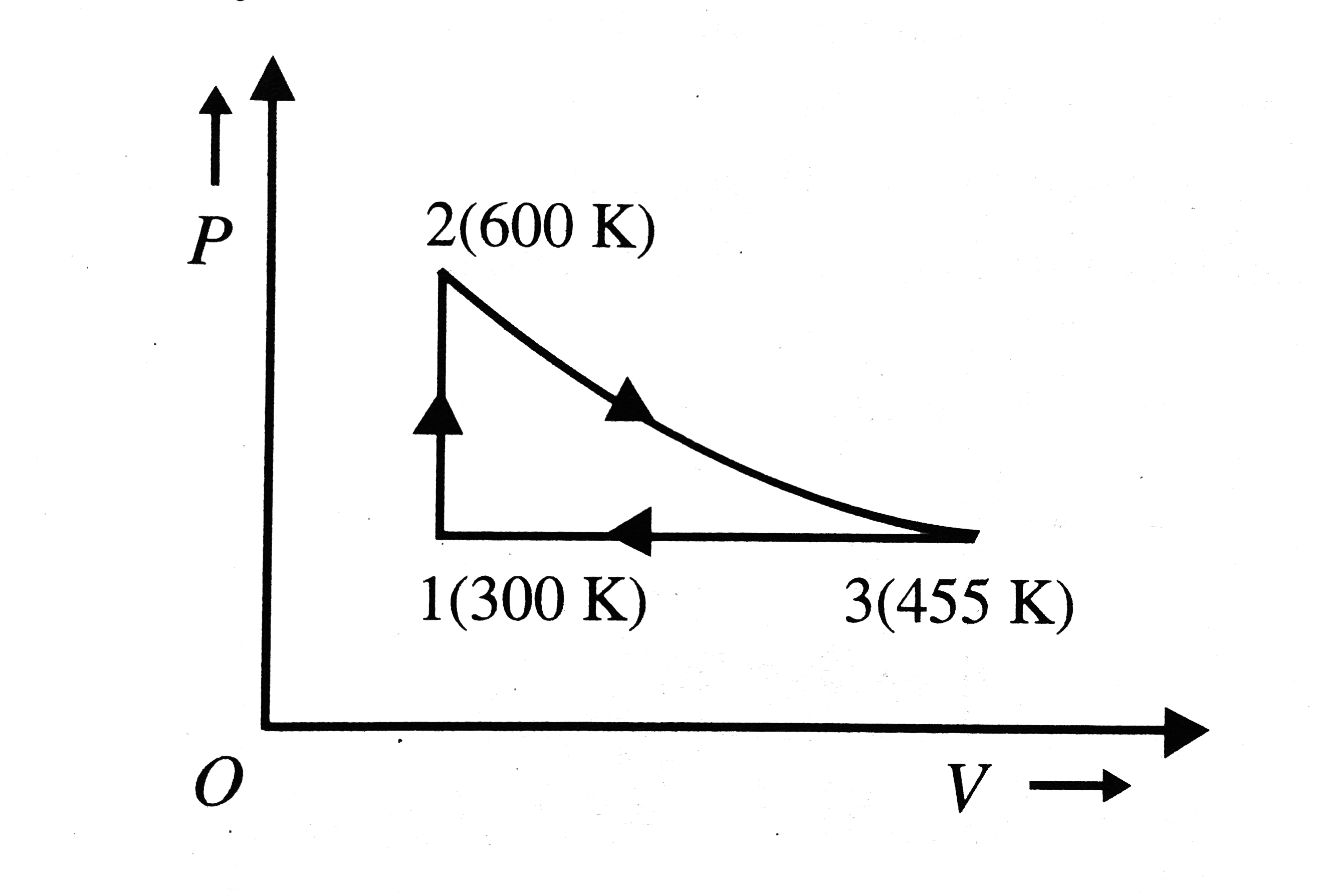
- A reversible heat engine carries 1 mole of an ideal monatomic gas arou...
Text Solution
|
- Two moles of an ideal gas are undergone a cyclic process 1-2-3-1. If n...
Text Solution
|
- A heat engine carries one mole of an ideal mono-atomic gas around the ...
Text Solution
|
- A reversible heat engine carries 1 mole of an ideal monatomic gas arou...
Text Solution
|
- Three processes compose a thermodynamic cycle shown in the accompanyin...
Text Solution
|
- A system undergoes three quasi-static process sequentially as indicate...
Text Solution
|
- One mole of helium gas follow cycle 1-2-3-1 shown in the diagram. Duri...
Text Solution
|
- A gas of adiabatic exponent gamma is supplied heat at a constant press...
Text Solution
|
- The temperature of 1 mol of an ideal monoatomic gas at constant pressu...
Text Solution
|