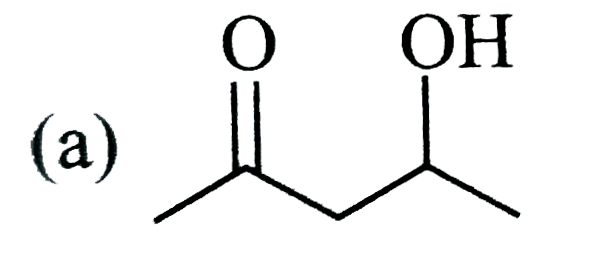
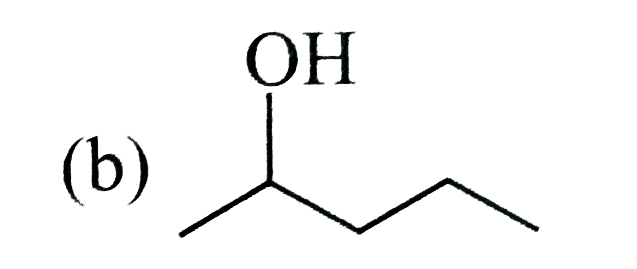
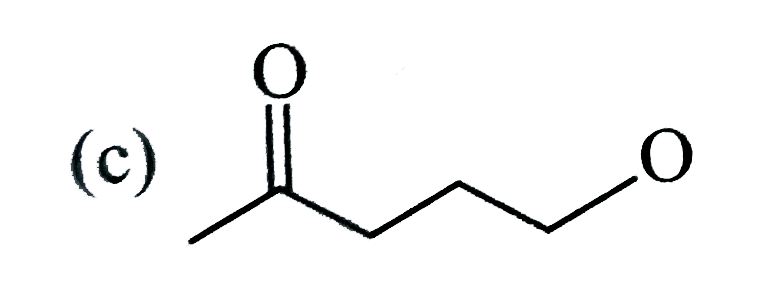
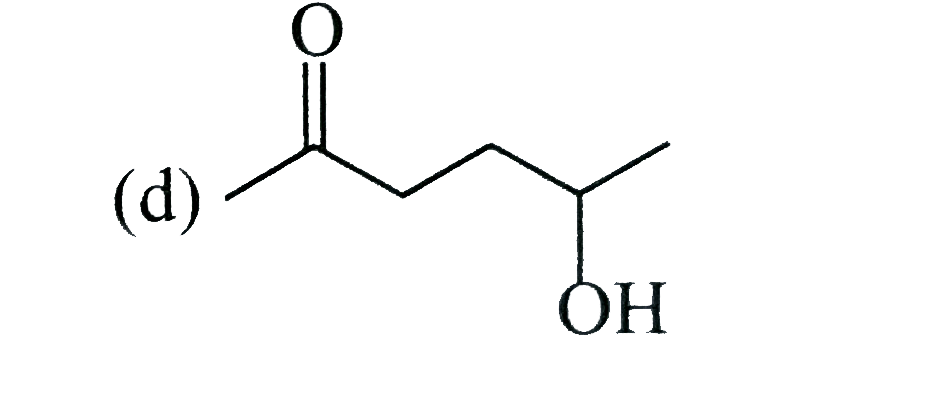
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACID
A2Z|Exercise Oxidation And Reduction Of Carbonyl Compounds|54 VideosALDEHYDES, KETONES AND CARBOXYLIC ACID
A2Z|Exercise Cannizaro'S Reaction|15 VideosALCOHOLS, PHENOLS AND ETHERS
A2Z|Exercise Section D - Chapter End Test|30 VideosBIOMOLECULES
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ALDEHYDES, KETONES AND CARBOXYLIC ACID-Section D - Chapter End Test
- Which one of the following will most readily be dehydrated in acidic c...
Text Solution
|
- Aldehydes with alpha-H atom do not undergo disproportionation because:
Text Solution
|
- Which of the following tests would help in the distinction of HCOOH an...
Text Solution
|
- In Cannizzaro reaction, which of the following is a better hydride don...
Text Solution
|
- Mark the correct order of increasing reactivity.
Text Solution
|
- Compound (A) given below can undergo Cannizzaro reaction itself and cr...
Text Solution
|
- CH3 CH=CHCHO is oxidised to CH3 CH=CHCOOH using :
Text Solution
|
- Clemmensen's reduction will convert cyclohexanone into:
Text Solution
|
- What of the following is expected to be most highly ionised in water ?
Text Solution
|
- Ethylidene chloride is hydrolysed with aqueous NaOH. The product forme...
Text Solution
|
- The vapours of ethyl alcohol are passed over red hot copper at 573 K t...
Text Solution
|
- (CH(3))(2) CHOH overset ("Mild") underset ("oxidation"[0]) to (X) over...
Text Solution
|
- The chemical reaction of acetaldehyde and ammonia results in:
Text Solution
|
- When diethyl cadmium [(C(2)H(5))(2)Cd] is treated with acetyl chloride...
Text Solution
|
- The product formed by the reaction of acetaldehyde with excess of etha...
Text Solution
|
- Which of the following will not be formed when calcium formate is dist...
Text Solution
|
- In order to prepare acetone from acetyl chloride in one step, which of...
Text Solution
|
- Which of the following reagents can help in separating a mixture of ac...
Text Solution
|
- The reagents used to convert
Text Solution
|
- Which of the following is the stronger acid?
Text Solution
|
- Of the following which compound is an acetal ?
Text Solution
|