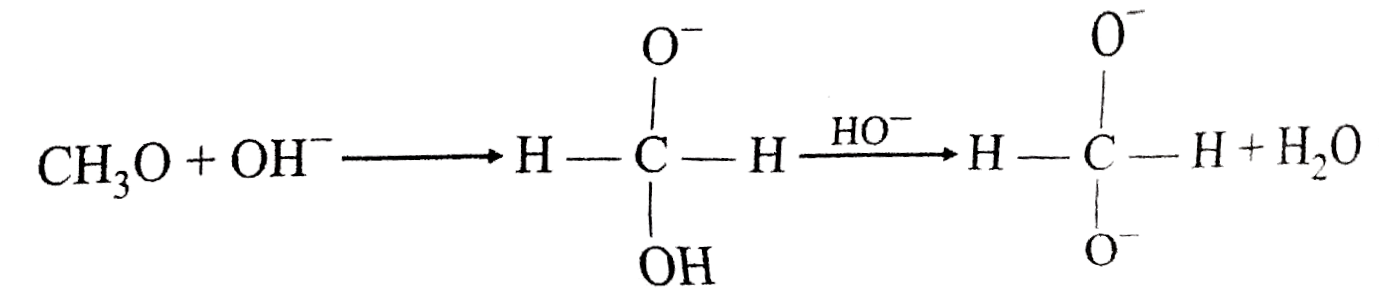A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACID
A2Z|Exercise Miscellaneous Reactions Of Aldehydes And Ketones|11 VideosALDEHYDES, KETONES AND CARBOXYLIC ACID
A2Z|Exercise Carboxylic Acid|59 VideosALDEHYDES, KETONES AND CARBOXYLIC ACID
A2Z|Exercise Oxidation And Reduction Of Carbonyl Compounds|54 VideosALCOHOLS, PHENOLS AND ETHERS
A2Z|Exercise Section D - Chapter End Test|30 VideosBIOMOLECULES
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ALDEHYDES, KETONES AND CARBOXYLIC ACID-Cannizaro'S Reaction
- The major organic product in the following reaction is
Text Solution
|
- Which one of the following undergoes reaction with 50% sodium hydroxid...
Text Solution
|
- The Cannizzaro reaction is not given by
Text Solution
|
- For a Cannizzaro reaction R - CHO + NaOH rarr Rate law is derived ...
Text Solution
|
- Trichloroacetalhyde was subjected to Canizzaro's reaction by using NaO...
Text Solution
|
- m-Chlorobenzaldehyde on reaction with conc. KOH at room temperature gi...
Text Solution
|
- In the Cannizzaro reaction given below: 2Ph-CHO overset(overset(Θ)OH...
Text Solution
|
- In a Cannizaro reaction the intermediate that will be the best hydride...
Text Solution
|
- Which m-pchlorobenzaldehyde is treated with 50% KOH solution, the prod...
Text Solution
|
- A mixture of benzaldehyde and formaldehyde on heating with aqueous NaO...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Benzaldehyde +NaOH rarr
Text Solution
|
- Benzyl alcohol and sodium benzoate is obtained by the action of sodium...
Text Solution
|
- If formaldehyde and KOH are heated, then we get
Text Solution
|
- What is the name of reaction when benzaldehyde changes into benzyl alc...
Text Solution
|