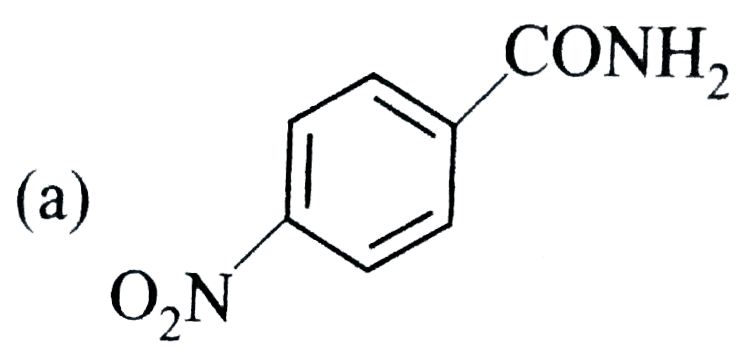
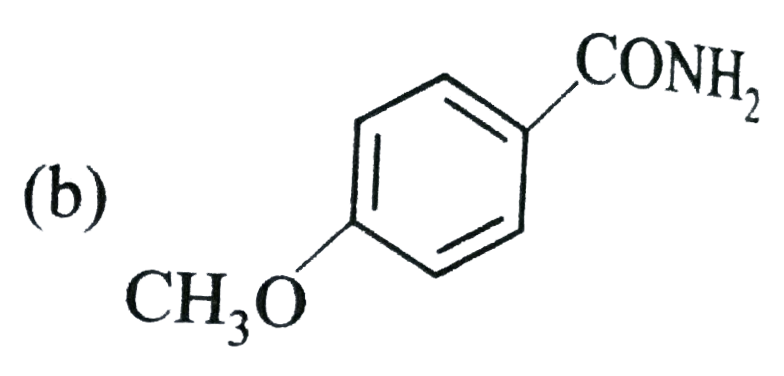
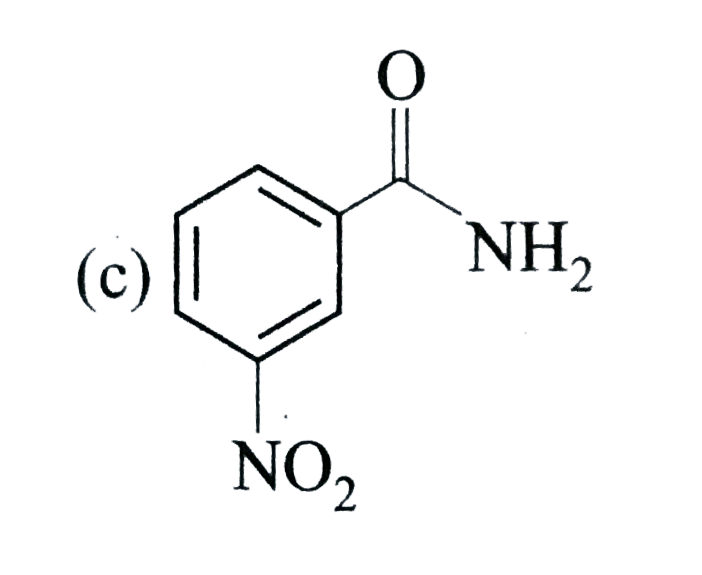
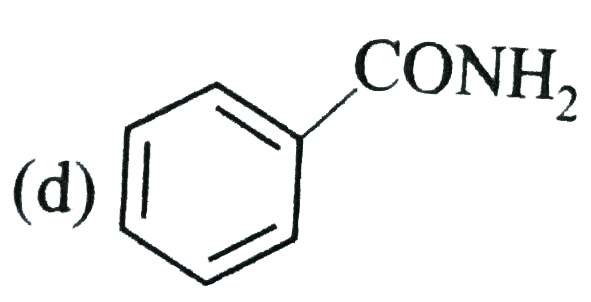
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACID
A2Z|Exercise Section A (Topicwise question|3 VideosALDEHYDES, KETONES AND CARBOXYLIC ACID
A2Z|Exercise Section B - Assertion Reasoning|33 VideosALDEHYDES, KETONES AND CARBOXYLIC ACID
A2Z|Exercise Carboxylic Acid|59 VideosALCOHOLS, PHENOLS AND ETHERS
A2Z|Exercise Section D - Chapter End Test|30 VideosBIOMOLECULES
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ALDEHYDES, KETONES AND CARBOXYLIC ACID-Derivatives Of Carboxylic Acid
- Which of the following ester is most likely to undergo unimolecular ac...
Text Solution
|
- What is formed as the major organic product in the reaction? CH(3)CH...
Text Solution
|
- Which is most reactive in base cataysed hydroysis reaction?
Text Solution
|
- Name the end product in the following series of reaction CH(3)COOH o...
Text Solution
|
- Which reaction given a polymeric amide?
Text Solution
|
- Benzoyl chloride is prepared from benzoic acid by :
Text Solution
|
- Ethyl ester overset(MeMgBr) underset(excess)rarr P. The product P will...
Text Solution
|
- In the reaction the structure of the product T is:
Text Solution
|
- The compound P, Q and S were separtely subjected to nitration usi...
Text Solution
|
- The order of decreasing raye of reaction with ammount is
Text Solution
|
- CH(3)COOCH(3) + "excess" PhMgBr rarr "product" overset (H^(+)) to X Th...
Text Solution
|
- When acetamide is treated with NaOBr, the product formed is
Text Solution
|
- Saponification of ethyl benzoate with saustic soda as alkali gives
Text Solution
|
- Acetyl chloride is reduced with LiAIH(4) the product formed is
Text Solution
|
- In the preparation of an ester, the commonly used dehydrating agent is
Text Solution
|
- Acetamid reacts with P(2)O(5) (phosphorus pontoxide) to give
Text Solution
|
- Acetamid is
Text Solution
|
- Acetic anhydride reacts with diethyl other in presence of anhydrous AI...
Text Solution
|
- The regent which does not give acid chloride on treating with a carbox...
Text Solution
|
- An oganic compound is boiled with alcoholic potash. The product is coo...
Text Solution
|