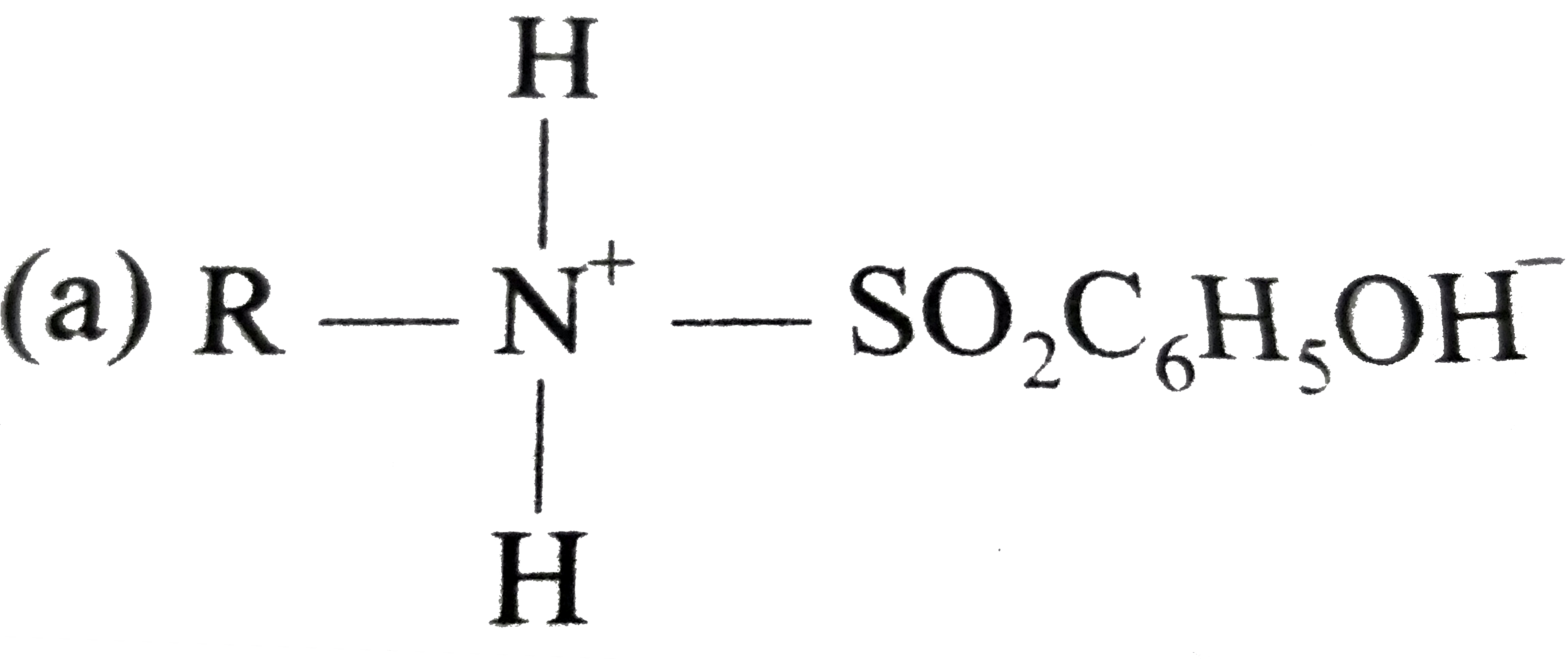
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Aromatic Amines And Diazonium Salts|61 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Nitrocyanide And Isocyanides|32 VideosORGANIC COMPOUNDS CONTAINING HALOGENS
A2Z|Exercise Section D - Chapter End Test|30 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ORGANIC COMPOUNDS CONTAINING NITROGEN-Section D - Chapter End Test
- RNH2 reacts with C6H5SO2Cl in aqueous KOH to give a clear solution. On...
Text Solution
|
- Which of the following nitro compounds will show tautomerism?
Text Solution
|
- Gabriel synthesis is used for the preparation of
Text Solution
|
- The major product of the reaction is:
Text Solution
|
- Which of the following statement is correct?
Text Solution
|
- Which of the following is the weakest base?
Text Solution
|
- Only one the following amines will lose its nitrogen atom as trimehyl ...
Text Solution
|
- Which of the following cannot react with HNO2?
Text Solution
|
- In order to distinguigh between C2H5NH2 and C6H5NH2 which of the fol...
Text Solution
|
- Which of the following amines reacts most rapidly with
Text Solution
|
- Which of the following produces one mononitro and three isomeric dinit...
Text Solution
|
- The conjugate base of (CH3)2NH2^(Theta) is:
Text Solution
|
- Identify major product of following sequency of reaction:
Text Solution
|
- Primary and secondary amines are distinguished by:
Text Solution
|
- The product of the reaction of alcoholic silver nitrite with ethyl bro...
Text Solution
|
- Identify the final product of following sequence of reaction:
Text Solution
|
- Tertiary nitro compounds cannot show tautomerism becauses :
Text Solution
|
- When C6H5N2Cl is reduced with Na2SnO2, the product is:
Text Solution
|
- Identify final product of following sequence of reaction:
Text Solution
|
- Which of the following substances on treatment with P2O5 gives ethanen...
Text Solution
|
- Which of the following reagents on treatment with benzenamine in basic...
Text Solution
|