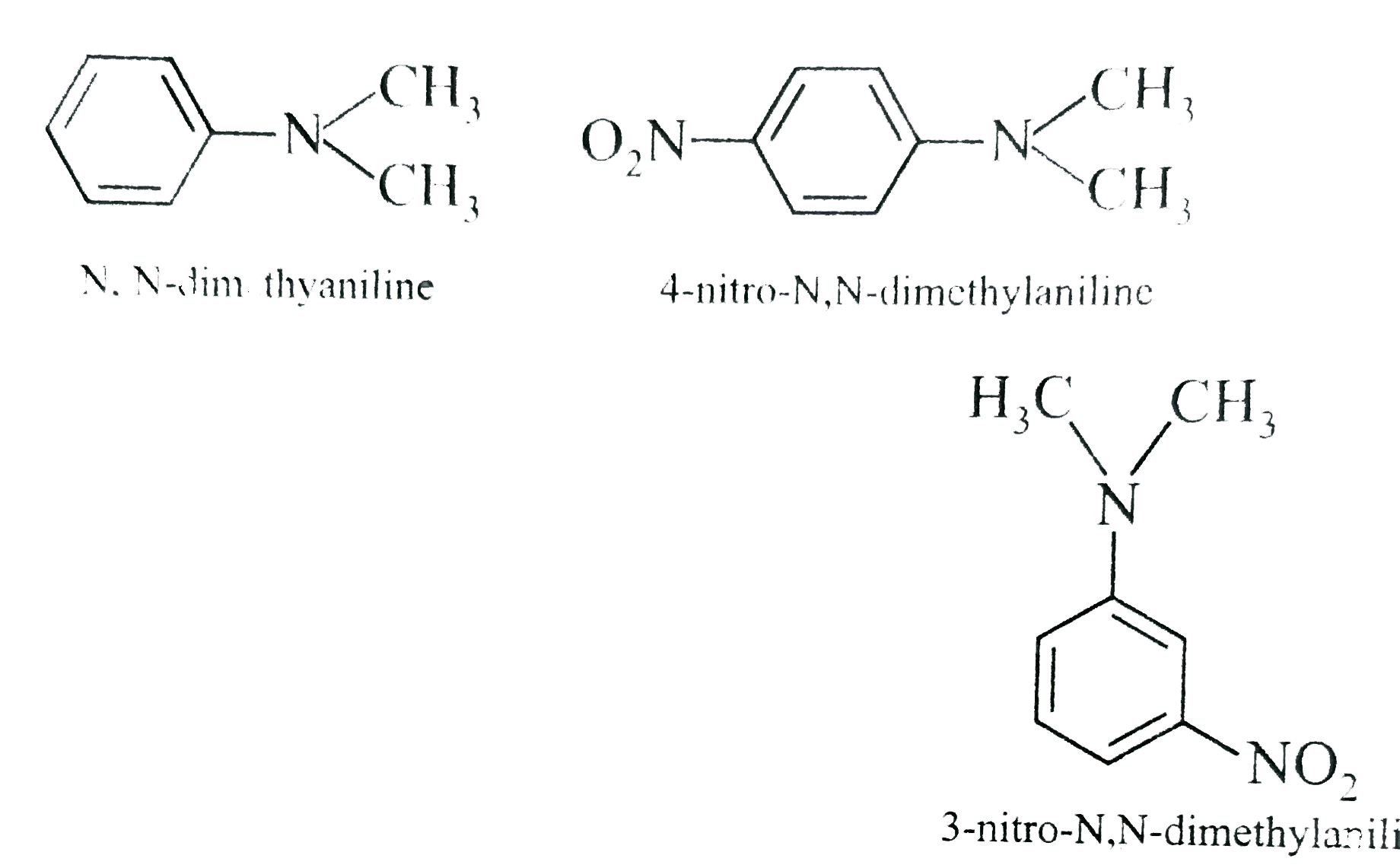A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Nitrocyanide And Isocyanides|32 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Section B - Assertion Reasoning|11 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Section D - Chapter End Test|30 VideosORGANIC COMPOUNDS CONTAINING HALOGENS
A2Z|Exercise Section D - Chapter End Test|30 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ORGANIC COMPOUNDS CONTAINING NITROGEN-Aromatic Amines And Diazonium Salts
- Aromatic nitriles (ArCN) are not prepared by reaction .
Text Solution
|
- Oil of mirbane is
Text Solution
|
- A student tried to synthesis 4-nitro-N, N-dimethylaniline from N, N-di...
Text Solution
|
- p-chloro aniline and anilinium hydrochloride can be distinguished by
Text Solution
|
- By reduction of mitrosobenzene which f the following is not obtained
Text Solution
|
- Among the following compounds nitrobenzene, benzene, aniline and pheno...
Text Solution
|
- An organic compound A has molecular formula C7H9N and it forms a clear...
Text Solution
|
- Aniline on treatment with excess of bromine water gives
Text Solution
|
- Which could not be directly prepared from 4-bromobenzenediazonium ion?
Text Solution
|
- When an organic compound was treated with sodium nitrite and hydrochlo...
Text Solution
|
- Derice a series of reaction of convert benzene into metachlorobromoben...
Text Solution
|
- Aniline reacts with alkyl halide to give
Text Solution
|
- Which of the following is regenerated at the end of the reaction?
Text Solution
|
- Which of the following compounds does not react with NaNO2 and HCl?
Text Solution
|
- An azo compoudn X is cleaved at the azo linkage by stannous chloride S...
Text Solution
|
- Which one is less alkaline?
Text Solution
|
- In the diazotasation of aniline with sodium nitrite and hydrochloric a...
Text Solution
|
- Aniline and methyl amine can be differentiated by
Text Solution
|
- Reaction of aniline with benzaldehyde is
Text Solution
|
- In the reaction C6H5CHO+C6H5NH2rarrC6H5N=HCC6H5+H2O and compound C6H5N...
Text Solution
|