A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Section B - Assertion Reasoning|11 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise AIPMT/NEET Questions|26 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Aromatic Amines And Diazonium Salts|61 VideosORGANIC COMPOUNDS CONTAINING HALOGENS
A2Z|Exercise Section D - Chapter End Test|30 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ORGANIC COMPOUNDS CONTAINING NITROGEN-Nitrocyanide And Isocyanides
- What sequency of reaction would best accomplish the following reaction...
Text Solution
|
- The rate nitration of tuluene in nitromethane with excess of HNO3 is c...
Text Solution
|
- Nitrobenzenen can be prepared from benzene by using a mixture of conc ...
Text Solution
|
- Product C of the given reaction is:
Text Solution
|
- The alkyl cynides when ydrolysed to the corresponding acid, the gas ev...
Text Solution
|
- Major product (s) is/are?
Text Solution
|
- Primary nitro compounds when react with HNO2 forms crystalline solids ...
Text Solution
|
- Nitrosobenzene can be isolated from nitrobenzene under
Text Solution
|
- Secondary nitro compounds when react with HNO2 forms crystalline solid...
Text Solution
|
Text Solution
|
- When methyl cyanide is hydrolysed in presence of alkali, the product i...
Text Solution
|
- Identify the product Z in the series CH3CNrarroverset(Na+C2H5OH)toXo...
Text Solution
|
- In the reduction of nitrobenzene, which of the following is the interm...
Text Solution
|
- In the reaction CH3CN+CH3MgIrarrAoverset((H2O)/(H^(+)))toB The compo...
Text Solution
|
- Aniline when treated with conc. HNO3 gives
Text Solution
|
- p-Nitrobromobenzene can be converted to p-nitroaniline by using NaNH2....
Text Solution
|
- The mojor product (70% to 80%) of the reaction between m-dinitrobenzen...
Text Solution
|
- The most unlikely representation of resonance structures of p-nitrophe...
Text Solution
|
- What is formed, when nitrobenzene is reduced using zinc and alkali?
Text Solution
|
- Decreasing order basicity is (1) CH3CONH2 (2) CH3CH2NH2 (3) Ph-C...
Text Solution
|
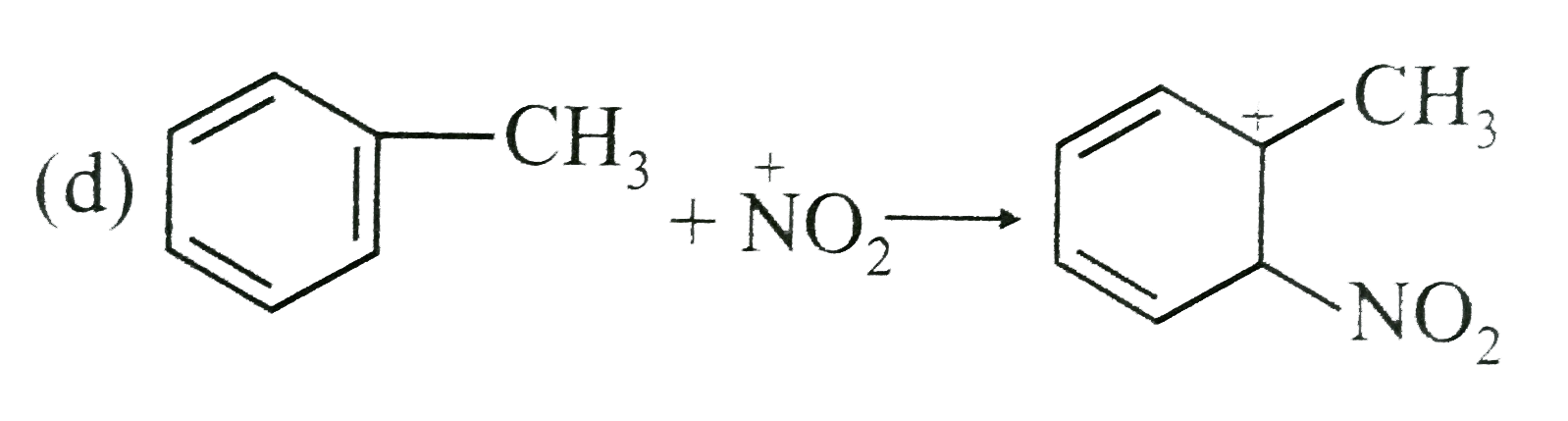
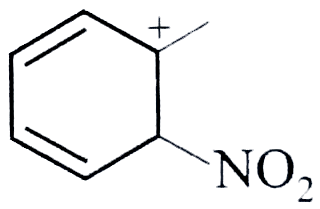 cannot be the rate determining step because the reaction is independent of the concetration of toluence.
cannot be the rate determining step because the reaction is independent of the concetration of toluence.