A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Section B - Assertion Reasoning|11 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise AIPMT/NEET Questions|26 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Aromatic Amines And Diazonium Salts|61 VideosORGANIC COMPOUNDS CONTAINING HALOGENS
A2Z|Exercise Section D - Chapter End Test|30 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-ORGANIC COMPOUNDS CONTAINING NITROGEN-Nitrocyanide And Isocyanides
- In the reduction of nitrobenzene, which of the following is the interm...
Text Solution
|
- In the reaction CH3CN+CH3MgIrarrAoverset((H2O)/(H^(+)))toB The compo...
Text Solution
|
- Aniline when treated with conc. HNO3 gives
Text Solution
|
- p-Nitrobromobenzene can be converted to p-nitroaniline by using NaNH2....
Text Solution
|
- The mojor product (70% to 80%) of the reaction between m-dinitrobenzen...
Text Solution
|
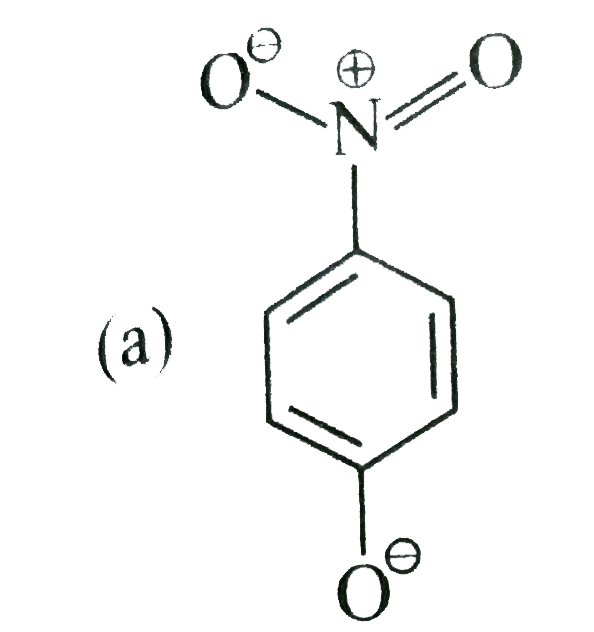
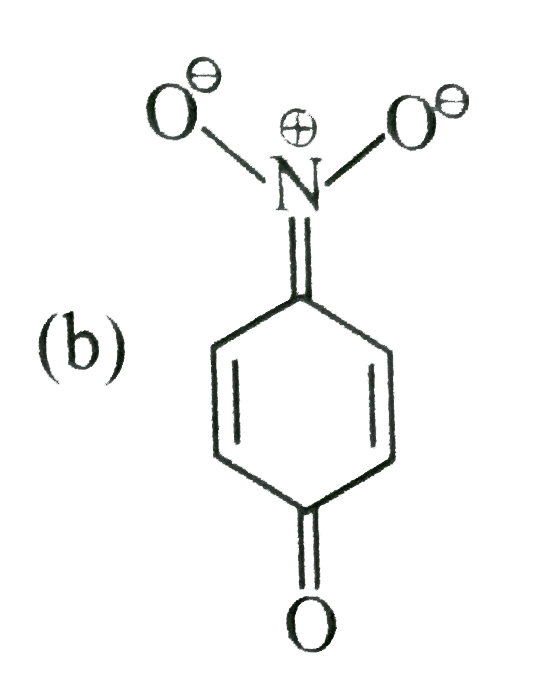
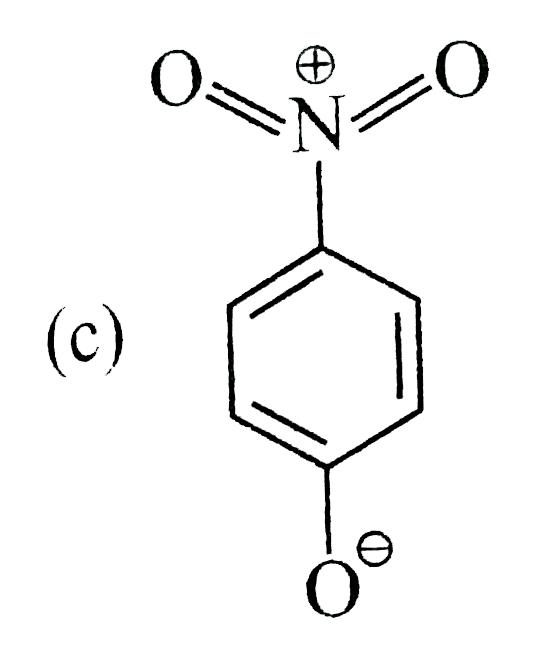
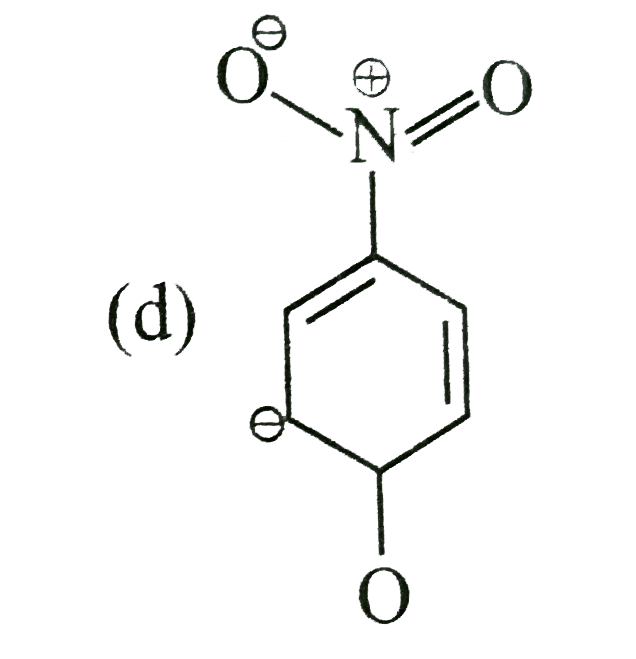
- The most unlikely representation of resonance structures of p-nitrophe...
Text Solution
|
- What is formed, when nitrobenzene is reduced using zinc and alkali?
Text Solution
|
- Decreasing order basicity is (1) CH3CONH2 (2) CH3CH2NH2 (3) Ph-C...
Text Solution
|
- Nitrobenzene on further excessive nitration gives
Text Solution
|
- Hydrolysisi of acetonitrile in acidic medium produces
Text Solution
|
- Product A in above reaction is :
Text Solution
|
- Product obtained by electrolytic reduction of nitrobenzene in presence...
Text Solution
|
- A nitrogen containing organic compound gave an oily liqid on heating w...
Text Solution
|
- in the reaction CH3CH+2Hunderset(Ether)overset(HCl)toXunderset(H2O)o...
Text Solution
|
- The following reaction is:
Text Solution
|
- Benzamide on reaction with POCl3 gives
Text Solution
|
- Nitrobenzene can be prepared from benzene by using a mixture of conc. ...
Text Solution
|
- The major product obtained when (Be2)/(Fe) is treated with
Text Solution
|
- In the following reactions, the major product W is:
Text Solution
|
- Presence of a nitro group in a benzene ring.
Text Solution
|